Biogenesis and function of centriolar
and ciliary structures
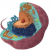
Paramecium cilia and basal bodies observed at photonic and electron microscopic level.
A: Confocal microscopy of Paramecium’s cilia labeled with antibodies specific to cilia (polyglutamylated tubulin antibodies-green) and cilia tips (monoglycylated tubulin antibodies-red) B: Longitudinal view of two basal body cortical unit. Only the posterior basal body is ciliated (mixed field) B’: Longitudinal view of two ciliated basal body unit (invariant field) C : Transversal section of a two basal body unit with its associated rootlets D: Schematic representation of the basal body duplication process. The duplication starts with the formation of a pro-basal body with its mother one anchored and ciliated at the cell surface. After elongation, the neo-formed basal body just tilt-up to anchor. Red arrows indicate the length of the transition zone (TZ)
Ciliogenesis is the culmination of a succession of spatially and temporally coordinated steps that may differ from one organism to another. However, it includes several key steps, common to all cellular models and conserved throughout evolution: duplication of the centriole/basal body, maturation of the basal body, migration and anchoring to the membrane leading to the formation of the transition zone and then growth of the cilium. In addition to a correct synthesis of the proteins involved, the assembly of this complex organelle depends on the correct protein-protein interactions in a 4-dimensional space-time and on their regulations by post-translational modifications such as ubiquitination/deubiquitination. Cilia are involved in many developmental and physiological processes and their alteration, in mammals, leads to physio-pathological disorders called “ciliopathies“. Otherwise, the loss of cilia observed in various tumors, reveals that ciliogenesis defects could be involved in some cancers.
Basal body anchoring defect after OFD1 depletion in Paramecium
Confocal microscopy of a wild type (A-A’) and an OFD1-depleted (B-B’) Paramecium stained with antibodies specific for basal bodies in white. A and B show the surface view of the ventral side of the cell. A’ and B’ show an optical section at the level of the cytoplasm. Note that in wild-type cells, all basal bodies are organized in longitudinal rows (A) and anchored at the cell surface (A’). In OFD1 depleted cells, basal bodies organization at the cell surface is altered (B) and unanchored basal bodies are found in the cytoplasm (B’)
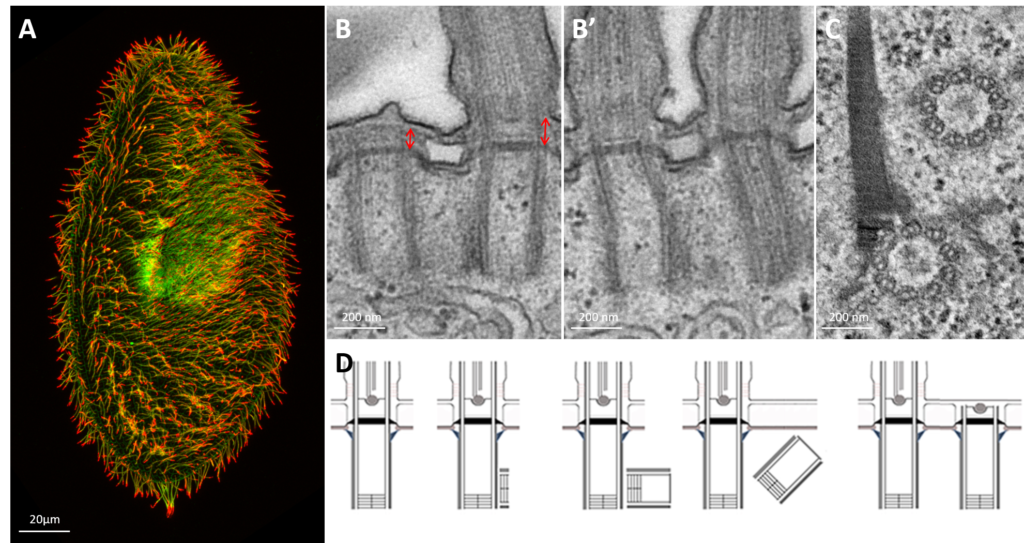
Paramecium Basal body unit purification
A: Paramecium cell surface is sustained by a cytoskeletal layer (the epiplasm) segmented in square (the cortical units) in the middle of which the basal bodies are inserted. B: fragment of Paramecium cortex B’: Dissociated cortical units used for Cryo-microscopy. C: Schema of a Paramecium basal body with its associated rootlets (from Jerka-Dziadosz et al, 2013): kinetodesmal fiber, transverse microtubules D: 2D view of Paramecium basal body observed by cryo-microscopy. The rootlets, kinetodesmal fiber and transverse microtubules, are observed. The basal bodies are well conserved.
The objectives of our team are multiple and are based on the synergy of experimental models : paramecium, mammalian cells and transgenic mice.
- to carry out the molecular and spatio-temporal dissection of the basal body anchoring step.
- to study the regulation of basal body anchoring by ubiquitination/deubiquitination processes by studying CYLD deubiquitinase and its partners
- to validate candidate genes involved in primary ciliary dyskinesias, human ciliopathies affecting motile cilia, using Paramecia as a study model
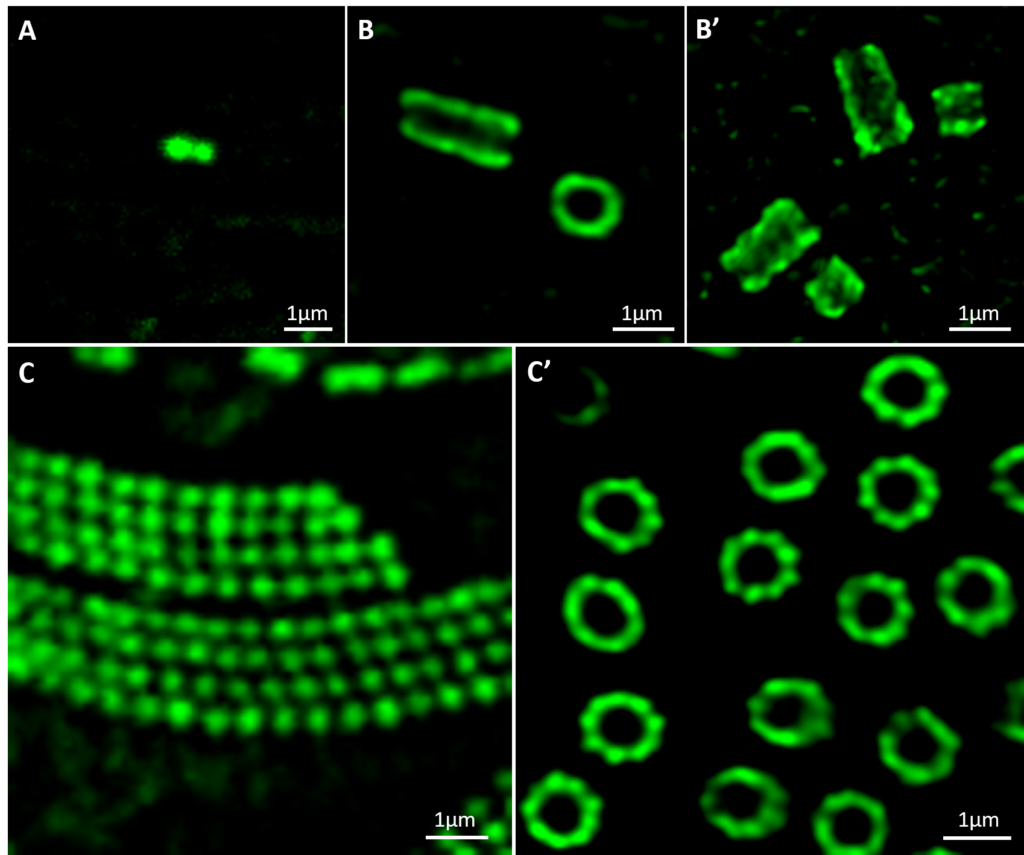
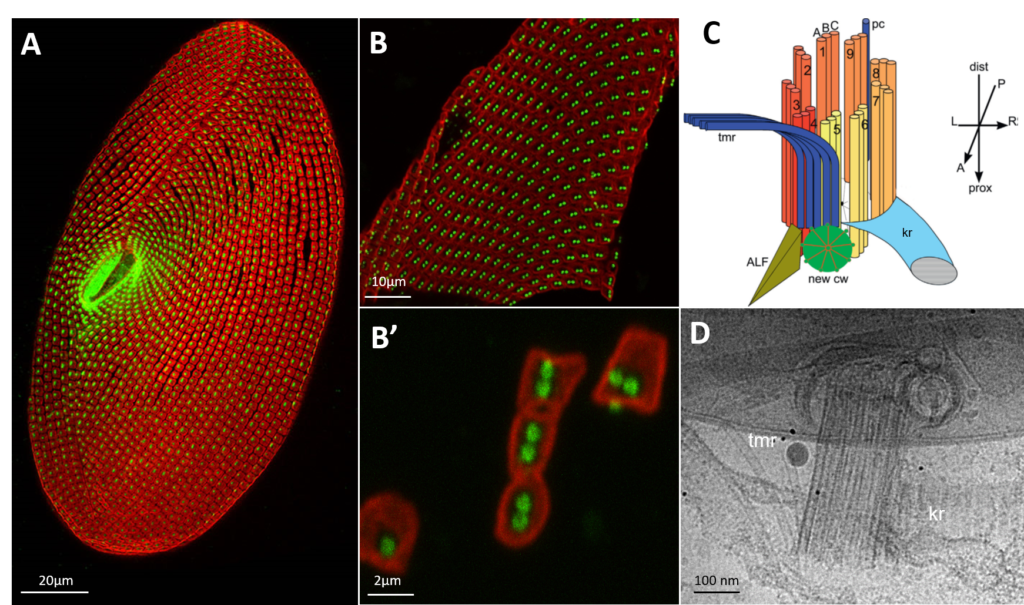
Paramecium transition proteins observed by STED microscopy
Representative STED images revealing distinct localization patterns of several GFP-tagged TZ proteins. Cells were labelled with anti-GFP or ID5(tubulin). A single ring differing in diameter is observed according to the observed protein. The mean diameters (distance between intensity maxima) and the number of BBs analyzed are given beneath each image, 2 replicates
Expansion microscopy UExM of human centrioles
and Paramecium basal bodies
Ultrastructural expansion-microscopy (U-ExM) of human centrioles (A’-B) and Paramecium basal bodies. B, B’ at different steps of centriole duplication. (C’) observed by confocal microscopy. A and C show human centrioles and Paramecium gullet pre-expansion observed by confocal microscopy.
team

Group Leader Senior Researcher

Lecturer

Researcher
Volunteer Researcher
Engineer
Technician
Engineer
team

Anne-Marie TASSIN
Group Leader
Research Director
Michel LEMULLOIS

Assistant professor
Khaled BOUHOUCH

Assistant professor
Karima PALMIER

Technician
Pierrick LE BORGNE

PhD
Logan GREIBILL

PhD
Cindy MATHON

Technician
Katia IGRANAISSI

Intern Student
Publications
For all the publications of the Team click on the button below.
External funding