Prot-Ex
Services
Small Secreted Proteins produced in Pichia pastoris
Expression of small secreted proteins in Pichia pastoris
The methylotrophic yeast P. pastoris is used to secrete in the medium small eukaryotic protein.
The users prepares themselves interested gene sub-cloning into pPIC9 or pPICZ plasmids.
The Platform accompanies the users to screen the producer clones,
We take in charge preparation of yeast cultivation using fermentors (DASGIP (4x1L) either in batch or in fed-batch mode.
Fermentation is followed by filtration, ultrafiltration and diafiltration steps of the supernatants on a Quixstand tool.
Quality control of the final protein production could be proposed.
Fermentation deliveries & bioreactors location
Rental of fermentation equipments on specific project. Please consult us.
Scientific manager: Karine Blondeau
Equipments

4 x 1 L Multi-Bioreactors (Dasgip)
5 L & 15 L Bioreactors (Applikon)
Tangential flow filtration and ultrafiltration QuixStand benchtop system
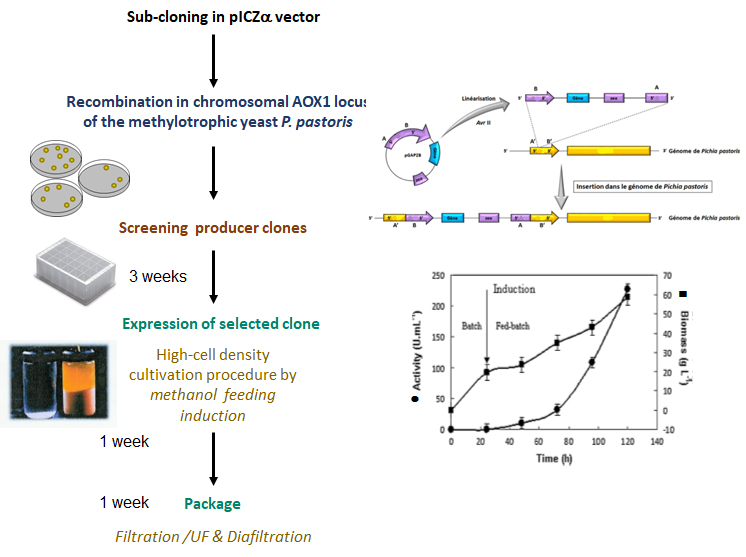
adapted from Multi-Copy Pichia Expression kit (2010) Invitrogen,
Proposals
Advices for screening producer clone
- Biomass and sample productions in high cell density cultivation (1L)
+ Supernatant preparations
Tangential flow micro & ultrafiltration in Hollow fiber filter module - Rental equipment
Production of Membrane Proteins in S. cerevisiae
Saccharomyces cerevisiae expression system
We use the baker yeast S. cerevisiae to express and co-express both, integral and membrane-associated proteins. The Platform proposes various volumes of production: from 0.5 to 20 L cultures using either, bioreactors or shaking flasks.
Users are in charge of cloning their proteins into a plasmid provided by us. This plasmid contains a strong inducible galactose promoter, and an ADE2 auxotrophic marker to allow growth at high density. Cell disruption and membrane fractionation can be provided by the Platform. Analysis of protein expression yield via Western-Blot is possible as well the assessment as protein solubility and homogeneity in detergent micelles using fluorescent reporters. Training new users to use the equipment is also possible
Scientific manager: José Luis VÁZQUEZ-IBAR
Technical managers: Cédric MONTIGNY and Céline RANSY
Equipments


- Thermoregulated Multitron (Infors HT), and Innova S44i (New Brunswick) shaker incubators.
- 1x 20L TechFor-S bioreactor (Infors HT).
- 2 x 1L MultiFor-S bioreactor (Infors HT).
- Beckman Avanti J20XP centrifuge with a JLA 8.1000 rotor (6 liters capacity)
- Planetary Mill for disruption of yeasts from 4 g to 1 kg « Pulverisette» (Fritsch).
- AKTA FPLC system (Cytiva) coupled with a JASCO Fluorescent detector.

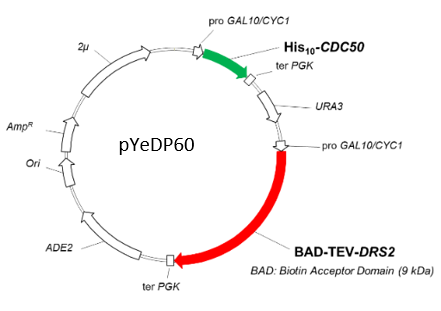
– Protein target is cloned by the users in the pYeDP60 vector
– Different tags are available (e.g., Biotin Acceptor Domain, Strep-tag, Fluorescent Proteins, His-tag)
– Three different
S. cerevisiae strains available
– From a few millilitersto 20 liters
– Protein expression yield
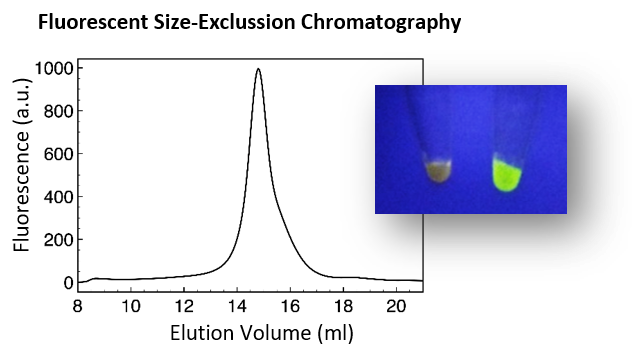
– Quality control by Fluorescent
Size-Exclusion Chromatography
Proposals
- Biomass production, cell disruption and membrane fractionation
- Quality control. Assessment of protein expression and solubility in detergent
In option.
Training new users to use the equipment independently is possible
Protein production in mammalian cells HEK
MAMMALIAN CELLS EXPRESSION
We use transient transfection of suspension cells using Expi293-GnTI (a commercial cell line derived from HEK293). This method can yield several milligrams of protein per liter within a 2-week period and produces a uniform glycosylation pattern. The costs are high (approximately 2000 € /L), making it primarily suitable for challenging cases.
Our team can also carry out transient transfection of adherent cells or establish stable-inducible cell lines (HEK-Flp-In Trex, which requires 1-2 months). These methods are not adapted to high-scale production
Scientific manager: François-Xavier THEILLET
Technical manager: Hayet IDRIS
Equipments

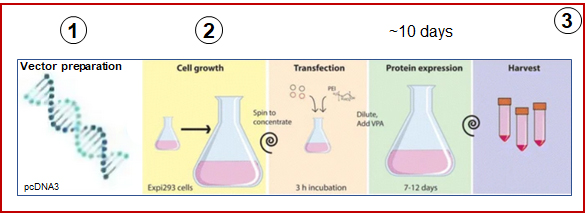
Fang, X.T et al. (2017), Biol Proced Online
- Cell line HEK- Expi293 GnTI
ThermoFisher/Gibco cells/media/transfection reagents - Cells in suspension with rotary shaker
Expression volume: 25 mL to 2 L
⇒ Multiple expression rounds to increase yield - Expression upon transient transfection
Plasmids with mammalian promoters
(e.g. pcDNA3 with CMV) - Generation of stable-inducible cell lines using adherent HEK-cells
Proposals
- Vector preparation
- Culture HEK Expi293 Gn-Ti
- Package : culture HEK293 &Transfection ExpiFectamine 293
Advantages
All mammalian proteins and protein-complexes
Homogeneous glycosylation
13C-Met labeling possible
Protein yields: ~0.5 to 50 mg/L
High reproducibility
Fast-track projects : 10 days
CELL FREE expression using Wheat germ extract
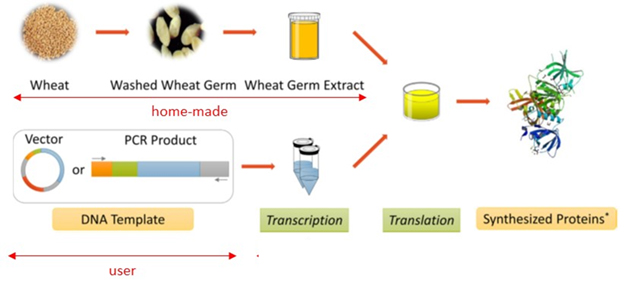
WHEAT GERM CELL FREE EXPRESSION SYSTEM
Cell-free expression systems are well suited for difficult-to-express proteins such as toxic and/or membrane proteins. The wheat germ extracts provide the highest translation efficiency among eukaryotic cell-free protein expression approaches and can produce on the order of 1 mg of purified protein from 1 mL of extract. In tight collaboration with Anja Böckmann from Lyon, we have implemented the wheat germ cell-free expression system in our laboratory. We can now accept 2-3 projects each year.
We provide users with the dedicated vector as well as advices to perform cloning steps (including the choice of preferable affinity tags). Transcription, translation and sample treatment are done by the platform staff. However, one person of the proposal can spend some days in the lab to participate to the experiments. Then, protein expression analysis is usually done by the users in their own lab but the platform can provide support. Since it is an open system, the translation reaction allows for addition of additives or cofactors such as detergents, liposomes, protein partners. Specific needs will be discussed.
The platform offers two types of experiments:
– small-scale protein expression tests
Is the protein expressed? Is it soluble? Solubilizing effect of detergents can be tested.
– medium- or large-scale expression
Production of protein using optimized conditions for efficient expression. The volume of extract depends on the expression yield. The platform is not intended to perform protein purification but can help users in the framework of a collaboration.
Scientific manager: Sonia FIEULAINE
Equipments

Prerequisite
Done by user prior to experiments
- Cloning in pEU-E01-MCS vector
- Maxiprep followed by phenol/chloroform extraction
Trowitzsch S. et al. (2010), Journal of Structural Biology
Proposals
- Screening tests (small-scale)
in 96-wells plates
± Solubilization tests
± Temperatures screening
Analysis by SDS-PAGE and Western-blot by the user. - Protein expression (large scale)
after expression tests in 96-wells plates (1 or more)
Protein purification is not included in the services and has to be done by the user, immediately after protein expression.
Baculovirus Expression Vector in insect cells Sf21 or H5
Spodoptera frugiperda ou Trichoplusia ni
INSECT CELLS EXPRESSION
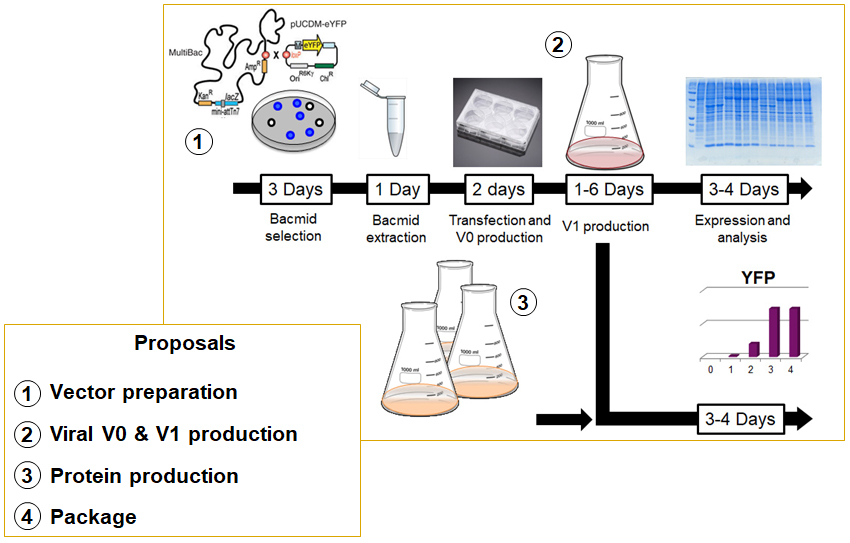
The MultiBac system, developed in collaboration with Imre Berger at the University of Bristol, is designed to produce high molecular weight multi-protein complexes. This system enables the concatenation of individual proteins and complexes in a stepwise or combinatorial manner, without any theoretical limit on the number of partners. Insect cells (Sf21 or Hi5) are cultivated in suspension in a rotary shaker, allowing up to 3L of production per unit. The YFP marker on the Bacmid is used as a protein production reporter. The production of YFP is followed on a FACS to stop the culture at the optimum of proteins production.
The cloning step into pKL, pFL (both provided by us), or pFastBac vectors is a prerequisite for the user. Quality control is conducted via SDS-PAGE and Western blot analysis. Note that protein purification is not included in our services and must be carried out by the user. One or two individuals may spend ten days being trained in insect cell production and achieving their first production using their biological systems.
Scientific manager: Virginie ROPARS
Technical manager: Hayet IDRIS
Equipments

- MultiBac system
implemented in collaboration with Imre Berger - Production of high Mw multi-protein complexes
- Insect cells cultivated in suspension with rotary shaker ( 6 x 600mL/ week)
⇒ Multiple expression rounds to increase yield
- YFP present on Bacmid as a protein production reporter
⇒ Production followed by FACS to stop culture at optimum protein production
Booking
Our Price are visible on the website GIPSI
Training
Master in microbiology, Paris Saclay University, “Industrial and microbial biotechnology”
Master in Biochemistry, Paris Saclay University, “Expression, Purification & characterization of membrane proteins
Bachelor Bioindustry & Biotechnologies, Paris Saclay University , “Membrane protein expression and purification”
Master, Protein Biochemistry, Pasteur courses, “Expression of protein of eucaryotic origin”
Engineer students, Polytech Angers, “Fermentation process engineering”
Master in applied microbiology, Paris City University, “Methodologic and strategic approaches in microbiology”
FORMATION & TRAINING
Prot-EX PLF propose training on fermentation equipments
SHORT TRAINING COURSES
Acknowledgements
Acknowledgements
Please acknowledge the protein expression platform of I2BC in your posters, presentations and articles by using the following sentence:
This work benefited from the crystallization platform of I2BC, supported by the French Infrastructure for Integrated Structural Biology (FRISBI) [ANR-10-INSB-05-05]
Citations
In case of co-authorship of platform members, please indicate this address
Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC), 91198, Gif-sur-Yvette, France

