Electron Microscopy
Equipments
All the equipments are accessible after training by the facility engineers.
MICROSCOPES

Zeiss Crossbeam 550
Date of installation: October 2022
Voltage: 0.5-30kV
SEM Column: Gemini2
FIB Column: Ion-Sculptor
Detectors: SE2 – InLens – EsB – BSD4 – STEM
Application software: SmartSEM – SmartFIB – Atlas5 – Atlas 3D Nanotomography

JEOL1400 (TEM)
Date of installation: June 2007
Voltage: 80-120 kV
Polar piece: High Contrast & High Tilt (Cs=3.2mm)
Camera: RIO9, 3kx3k, 9 µm/pixel, 15 images/sec (Gatan)
Holders:
- Multi-holder (JEOL specimen quartet holder)
- High-tilt holder (JEOL)
- High-tilt holder double tilt (Gatan 927)
JEOL1400 (TEM and STEM)
Date of installation: December 2011
Voltage: 80-120 kV
Polar piece: STEM STEM High Contrast & High Tilt (Cs=2.1mm)
Camera: RIO9, 3kx3k, 9 µm/pixel, 15 images/sec (Gatan)
Detectors:
HADF detector (Bright field and dark field STEM)
EDXS detector (Numérix, SamX)
Holders:
- Multi-holder (JEOL specimen quartet holder)
- High-tilt holder (JEOL)
- High-tilt holder double tilt (Gatan 927)
- Beryllium holder (JEOL) for EDXS analysis
- Cryo-holder (Gatan 626), cold stage controller and pumping station (Gatan)
SAMPLE PREPARATION

The Leica EM PACT2 is a high-pressure freezing system for vitrifying samples up to 200µm in thickness without the artefacts of chemical fixation.
The Leica EM PACT2 makes it possible to observe aqueous biological and industrial samples near to native state by preserving the high-resolution information of EM immunocytochemistry, frozen hydrated sections, and freeze fractured samples.

The Leica EM AFS2 performs:
- Freeze substitution after high pressure freezing
- Progressive lowering of temperature (PLT) techniques
- Low temperature embedding and polymerization of epoxy and acrilic resins.
The Leica EM FSP (freeze substitution processor), an automatic reagent handling system combined with the Leica EM AFS2, dispenses reagents for both freeze substitution and PLT applications.
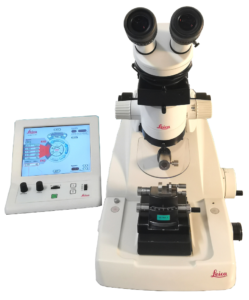
Leica EM UC6 allows the easy preparation of 50 to 200nm thin sections for TEM imaging. A heating plate and an optical microscope are available to check the quality of the sample via semi-thin sections.
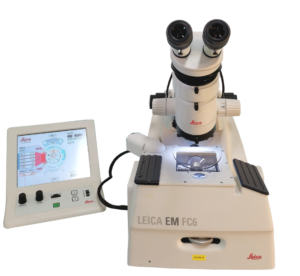
The Leica EM FC6 coupled to the UC6 allows routine ultrathin frozen sectioning. A cryo-chamber surrounding the sectioning area is cooled (from -80°C to -150°C) by liquid nitrogen pumped from a Dewar flask.

The Leica EM IGL allows the simultaneous labelling of 24 grids as well as the ability to use up to 24 different primary antibodies in one run. Automation with the EM IGL saves 80% of the users time. Specimen safety is ensured by applying the correct sequence of reagents and minimizing cross contamination from forceps and loops during labelling.
IMAGE ANALYSIS

The electron microscopy facility put in place image analysis software for image processing, tomography and 3D reconstruction :
- GMS3 (Gatan)
- Imod (Boulder, Colorado)
- Ilastik
The computer are equiped with a graphic tablet (Wacom CINTIQ pro 24) to facilitate the segmentation process.
Rates
Training
Training in Electon Microscopy
Contact us for more informations
Electron Microscopy applied to cell biology
Imagerie-Gif EM facility proposes training on all its equipment all along the year.
Specific training are proposed once a year to learn electron microscopy applied to cell biology :
Services
All the equipment are accessible after training by the facility’s engineers.
The facility engineers can perform experiments for the external users.
When the implication of the engineer is consistent, it has to be included in the authorship of the publication.

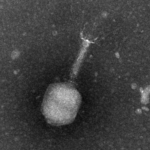
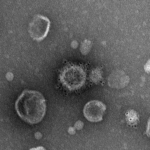
Negative staining or direct observation (nanoparticles, virus, purified vesicles)
Negative staining is a method for contrasting a thin specimen with electron-dense stain (usually heavy metal salts). In this technique, the background is dark, leaving the specimen in bright. This technique is suitable for small particles (10-500nm). If the particles are naturally electron-dense, the staining step is not necessary, and the sample is dried on a grid.
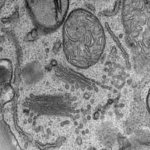
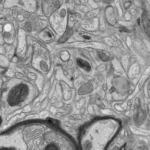
Resin embedding for ultrastructural analysis
Biological samples are fixed by chemicals or high pressure freezing (EMPACT2) and embedded in resin.
Then, we can produce thin section with an ultramicrotome (UC6) to observe them in a TEM.
This technique allows morphological analysis of cells or tissues at the nanometer resolution.
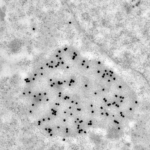
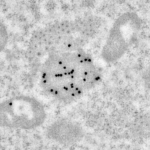
Immunolabeling
Immunodetection are processed according to 3 modalities:
- Immunolabeling on purified small particles: the antibodies are applied on purified particles and are then observed by negative staining in TEM.
- Immunolabeling on resin section: cells or tissue are embedded in acrylic resins to allow the fixation of antibody on the section. Sections are then observed in the TEM.
- Immunolabeling on cryo-section: samples are sectioned at low temperature to avoid resin and improve the immunodetection. Cryo-sections are processed with an cryoultramicrotome (FC6), the immunolabeling is processed at room temperature and observed in the TEM.
Contact
Plateforme de Microscopie Electronique / Electron Microscopy Facility
Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC)
1 Rue de la Terrasse
91198, Gif-sur-Yvette, France.
BOULOGNE Claire, Head of facility, Research Engineer
Mail : claire.boulogne@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 50
GILLET Cynthia, Engineer
Mail : cynthia.gillet@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 50
DUPAS Cynthia, Technician
Mail : cynthia.dupas@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 50
Plateforme de Microscopie Electronique / Electron Microscopy Facility
Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC)
1 Rue de la Terrasse
91198, Gif-sur-Yvette, France.
BOULOGNE Claire, Head of facility, Research Engineer
Mail : claire.boulogne@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 50
GILLET Cynthia, Engineer
Mail : cynthia.gillet@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 50
DUPAS Cynthia, Technician
Mail : cynthia.dupas@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 50
Acknowledgements
Funding is essential for the proper functioning and development of the Imagerie-Gif facilities. To this end, it is essential to acknowledge the facility as soon as you have benefited from the help of the engineers or any equipment.
Use this sentence to acknowledge the Electron Microscopy Core Facility :
The present work has benefited from Imagerie‐Gif core facility supported by l’Agence Nationale de la Recherche (ANR-10-INBS-04/FranceBioImaging ; ANR‐11‐IDEX‐0003‐02/ Saclay Plant Sciences )
Use this address for the Electron Microscopy Core Facility :
Electron Microscopy Facility, Imagerie-Gif, Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC), 91198, Gif-sur-Yvette, France.
