Light Microscopy
Equipments
All the equipments are accessible after training by the facility engineers.
Super-Resolution Microscopy

Microscope body
Inverted Eclipse Ti-E (Nikon)
Piezo Z-stage, Nano Z100-N (MCL)
Perfect focus System (3rd generation) (Nikon)
Sources
Lasers
405 nm (Cube, 100 mW, Coherent)
488 nm (Sapphir, 150 mW, Coherent)
561 nm (Sapphir, 150 mW, Coherent)
647 nm (MPB Communication, 300 mW)
Lamp
Intensilight mercury
lamp (130 W, Nikon)
Objectives
20x PL APO oil immersion (N.A : 0.75) (Nikon)
100x APO TIRF SR oil immersion (N.A : 1.49) (Nikon)
Filter Blocks
DAPI : BP 377/50, DM 409 nm, BP 430/45 (Nikon, Semrock 2SKZ4DAPIB)
FITC : BP 482/35, DM 506 nm, BP536/40 (Nikon, Semrock 2SZK4FITC0)
TRITC : BP 543/22, DM 562 nm, BP 593/40 (Nikon, Semrock 2SKZ4TRITC)
QUAD : 405/488/561/647 (Nikon, Chroma)
TRIPLE : 405/488/561 (zt405/488/561rpc-UF2) (Nikon, Chroma)
Camera
Andor iXon Ultra 897 EM-CCD (pixel size : 16×16 µm)
TIRF
Motorized Tirf arm (TI-TIRF-E ) (Nikon)
Software
NIS-Elements Advanced Research

Microscope body
Inverted AxioObserver Z1 / 7 (Zeiss)
Motorized stage
Piezo focus WSB 500
Focus controller : Definite Focus 3 (Zeiss)
Incubator components : incubation chamber XL, (CO2, temperature controllers (OKO Lab))
Correlative module (FIB-SEM sample holder + navigation software)
Sources
Lasers
HR Diode 405nm/50mW
HR Diode 488nm/100mW OPSL
HR DPSS 561nm/100mW OPSL
HR Diode 642nm/150mW
Lamp
Reflected light – Fluo HXP 120V (Zeiss)
Transmitted light – Halogen Lamp 12V/100 W
Objectives
10x EC Plan-Neofluar NA 0.3 Dry / Apotome SIM
20x Plan-Apochromat NA 0.8 Dry / Apotome SIM
40x Plan-Apochromat NA 1.4 Oil / DIC / (UV) / VIS-IR / Apotome SIM
63x Plan-Apochromat NA 1.4 Oil / DIC / Lattice SIM)
Filter Blocks
Reflector
Filter set 25 DAPI / FITC / Texas Red Wide Field
Filter Set LBF 405 / 488 / 561 / 642 BGOR (Blue / Green / Orange / Red)
Filter Set BP 420-480 / BP 495-550 / LP 650 BGR (Blue / Green / Red)
Filter Set BP 495-550 / BP 570-620 GO (Green / Orange)
Filter Set BP 420-480 / LP 655 BR (Blue / Red)
Filter wheel FLEX
Filter wheel PURE
Camera
Monochrome sCMOS PCO.edge 4.2 CLHS with chiller LCS-BU
2048 x 2048 pixels
Pixel size 6.5 µm x 6.5 µm
Dynamic range 16 bits
SIM
Lattice SIM
5 Grids (G6 23µm; G5 27.5µm; G4 32µm; G3 36.5µm; G1 42µm)
Leap mode + burst mode
SIM² Processing
Software
Carl Zeiss Zen Black version 16.0.13.306
Acquisition + Processing options (Tiles, SIM, SIM2, Shuttle & Find)
Carl Zeiss Zen Blue 3.5.093 – Processing only
Laser scanning confocal microscopy
Microscope body
Inverted DMi 6000 (Leica)
Motorized stage
Sources
Lasers
Diode 405 nm (50mW)
Laser 488 nm OPSL (20mW)
Laser 552 nm OPSL (20mW)
Diode 638 nm (25mW)
Lamp
120 W Metal Halide lamp for fluorescence – EL6000 (Leica)
Halogen Lamp for transmission mode (Leica)
Objectives
10x HCX PL FLUOTAR dry (NA: 0.3) (∞/ – / D) (Leica)
20x HC PL APO CS2 multi-immersion (oil, glycerol, water) (NA: 0.75) (∞/ – / D) (0.17-W-0)
40x HC PLAN APO CS oil immersion (NA: 1.3) (∞/ 0.17/D) (Leica)
63x HC PLAN APO CS2 oil immersion DIC (NA: 1.4) (Leica))
Filter Blocks
A filter block: BP 360/40, DM 400 nm, LP 425 (Leica)
L5 ET filter block: BP 480/40, DM 505 nm, BP 527/30 (Leica)
N3 ET filter block: BP 546/12, DM 565 nm, BP 600/40 (Leica)
Y5 ET filter block: BP 620/60, DM 660 nm, BP 700/75 (Leica)
Detectors
2 PMTs (Hamamatsu 6357 multi-alkali)
1 PMT Trans
Complementary modules
Dye finder licence
Tiles Scan & Mark and Find
Software
Leica Application Suite X (LAS X) 3.5.7
Microscope body
Upright DM 6000 CS (Leica)
Motorized stage
Super Z-galvo stage
Light Sources
Lasers
Diode 405 nm (50 mW)
Laser Argon 458 nm (10mW), 476 nm (10mW), 488 nm (20mW), 496 nm (5mW), 514 nm (20mW)
Laser DPSS 561nm (20mW)
Laser HeNe 594 nm, 633nm (10mW)
Lamp
Metal Halide Lamp for fluorescence – EL6000 120W (Leica)
Halogen Lamp for transmission mode (Leica)
Objectives
10x HC PL FLUOTAR dry (NA: 0.3) (Leica)
20x HC PL APO CORR CS2 dry + DIC (NA: 0.75) (Leica)
63x HC PLAN APO CS2 oil immersion + DIC (NA: 1.4) (Leica)
Filter Blocks
A4 Filter Set: BP 360/40, DM 400 nm, BP 470/40 (Leica)
GFP Filter Set: BP 470/40, DM 500 nm, BP 525/50 (Leica)
N21 Filter Set: BP 515-560, DM 580 nm, LP 590 (Leica)
Scanning system
Classic galvo scanner 10 to 1 000 Hz (7 frames/sec en 512×512)
AOBS System
Detectors
1 PMTs (Hamamatsu, 6357 multi alkali)
2 GaAsP Hybrid (Hamamatsu)
1 PMT Trans
Complementary modules
Dye finder
FRAP-FRET
3D reconstruction
Tiles scan &Mark and Find experiment
Software
Leica Application Suite X (LAS X) 3.5.7

Microscope body
Inverted DMi 6000 (Leica)
Motorized stage
Super Z-galvo stage
Light sources
Lasers
Diode 405 nm (Leica, 50 mW)
Pulsed Diode 440 nm LDH-P-C-440B (0.8 à 4.0 mW @40MHz) (Picoquant)
White light laser 2 (80 MHz, from 470 to 670 nm) + pulse-picker
Lamp
120 W Metal Halide lamp for fluorescence – EL6000 (Leica)
Halogen Lamp for transmission imaging (Leica)
Objectives
10x HC PL FLUOTAR dry (NA: 0.4) (Leica)
20x HC PL APO CORR CS2 multi-immersion (oil, glycerol, water) (NA: 0.7) (Leica)
25x HCX IR APO water + DIC (NA: 0.95) (Leica)
40x HC PLAN APO CS oil immersion + DIC (NA: 1.3) (Leica)
63x HC PLAN APO CS2 oil immersion + DIC (NA: 1.4) (Leica)
Filter Blocks
A Filter Set: BP 360/40, DM 400 nm, LP 425 (Leica)
I3 Filter Set: BP 460/20, DM 510 nm, LP 515 (Leica)
N3 Filter Set: BP 546/12, DM 565 nm, BP 600/40 (Leica)
Scanning system
Classic galvo scanner 10 to 1 000 Hz (7 frames/sec en 512×512)
Resonant scanner 8000 Hz (28 frames/sec en 512×512
AOBS System
2 PMTs (Hamamatsu 6357 multi alkali)
2 GaAsP Hybrid (Hamamatsu)
1 PMT Trans
Complementary modules
Excitation and emission spectra
Dye finder
FRAP-FRET
3D reconstruction
Falcon (FLIM + Phasor)
Navigator
Software
Leica Application Suite X (LAS X) 3.5.6.
Spinning Disk confocal microscopy

Microscope body
Inverted Eclipse Ti-E (Nikon)
Sources
Lasers
405 nm, 100mW (Vortran)
488 nm, 150mW (Vortran)
561 nm, 100mW (Coherent)
642 nm, 100mW (Vortran)
Lamp
Intensilight mercury lamp (Nikon)
Objectives
10x PLAN APO dry objective (NA: 0.45) (Nikon)
40x PLAN FLUOR oil immersion objective (NA: 1.30) (Nikon)
60x APO TIRF oil immersion objective (NA: 1.49) (Nikon)
100x PLAN APO oil immersion objective (NA: 1.40) (Nikon)
100x APO TIRF oil immersion objective (NA: 1.49) (Nikon)
Filter Blocks
DAPI Filter block: BP 360/40, DM 400 nm, BP 460/50 (Nikon)
BV2A Filter block: BP 420/40, DM 455 nm, LP 470 (Nikon)
GFP BP Filter block: BP 470/40, DM 495 nm, BP 520/35 (Nikon)
GFP LP Filter block: BP 472/30, DM 505 nm, LP 510 (Nikon)
YFP Filter block: BP 500/20, DM 515 nm, BP 535/30 (Nikon)
TxRed Filter block: BP 560/40, DM 595 nm, BP 630/60 (Nikon)
CY5 Filter block: BP 620/60, DM 600 nm, BP 700/75 (Nikon)
Camera
ORCA-Flash4.0 LT CMOS camera (Hamamatsu) with a 6.5 μm × 6.5 μm pixel size
Prime 95B sCMOS camera (Photometrics) with a 11µm x 11µm pixel size
Spinning Disk
CSU-X1-A1, Nipkow Spinning Disk confocal system (Yokogawa)
Dichroic Mirrors
Single band dichroic mirror 491 nm (Semrock)
Dual band dichroic mirror 491/561 nm (Semrock)
Quad band dichroic mirror 405/491/561/641 nm (Semrock)
Emission Filters (for Spinning Disk part)
Bandpass 447/60 nm (Semrock)
Bandpass 525/45 nm (Semrock)
Bandpass 590/20 nm (Semrock)
Bandpass 607/36 nm (Semrock)
Bandpass 692/40 nm (Semrock)
Quad bandpass 440/40 nm, 521/20 nm, 607/34 nm, 700/45 nm (Semrock)
Longpass 420 nm (Semrock)
Longpass 500 nm (Semrock)
Azimuthal TIRF
iLas 2 module (GATACA Systems)
Dichroic Mirrors
Triple band dichroic mirror 405/491/561 nm (Semrock)
Quad band dichroic mirror 405/491/561/640 nm (Semrock)
Emission Filters
Bandpass 525/50 nm (Chroma)
Bandpass 605/52 nm (Chroma)
Bandpass 700/75 nm (Chroma)
FRAP
iLas 2 module (GATACA Systems)
Dual View
For the simultaneous observation of GFP and mCherry, we used Dual View DV2 (Photometrics), an emission splitting system, with a spectral module (Chroma) made of a dichroic mirror 565 nm and band-pass filters for GFP (ET525/50m) and mCherry (ET630/75m).
Super-resolution
Live SR module (GATACA Systems)
Software
MetaMorph software version 7.7 (Molecular Devices)

Microscope body
Inverted Eclipse Ti2-E (Nikon)
Piezo Z-stage, Nano Z200-N, 200 µm course (MCL)
Perfect focus System (Nikon)
Cage incubator for temperature and CO2 control (OKO Lab)
Sources
Lasers
405 nm, 100mW (Vortran)
488 nm, 150mW (Vortran)
561 nm, 150mW (Coherent)
Lamp
SOLA Light engine SE II (Lumencor)
Objectives
20x Plan Apochromat Lambda dry objective (NA: 0.75) ∞/0.17 WD 1 (Nikon)
60x Apochromat TIRF oil immersion objective (NA: 1.49) ∞/0.13-0.21 WD 0.12 Temp. Corr. Ring (Nikon)
100x Apochromat TIRF oil immersion objective (NA: 1.49) ∞/0.13-0.20 DIC N2 WD 0.12 Temp. Corr. Ring (Nikon)
Filter Blocks
DAPI Filter Set: BP 377/50, DM 409 nm, BP 447/60 (Semrock)
GFP Filter Set: BP 482/35, DM 506 nm, BP 536/40 (Semrock)
RFP Filter Set: BP 543/22, DM 562 nm, BP 593/40 (Semrock)
Camera
Prime 95B sCMOS camera (Teledyne Photometrics) with a 11µm x 11µm pixel size
Spinning Disk
CSU-W1-T1, Nipkow Spinning Disk confocal system (Yokogawa)
Dichroic Mirror
Quad band Zt 405/488/561/638 (Chroma)
Emission Filters
Bandpass 450/50 nm (Chroma)
Bandpass 525/50 nm (Chroma)
Bandpass 590/33 nm (Chroma)
Azimuthal TIRF
iLas 2 module (GATACA Systems)
Dichroic Mirror
Quad band Zt 405/488/561/638 (Chroma)
Emission Filters
Bandpass 450/50 nm (Chroma)
Bandpass 525/50 nm (Chroma)
Bandpass 590/33 nm (Chroma)
FRAP
iLas 2 module (GATACA Systems)
Software
MetaMorph software version 7.10.3.279 (Molecular Devices)
Widefield microscopy

Microscope body
Upright Nikon Eclipse 80i
Light sources
Lamp
Intensitilight C- HGFI _ Nikon 130 W Metal Halide lamp
HAL 100_Nikon Halogen lamp for transmission imaging
Objectives
10x Nikon Plan Fluor Dry (NA 0.3) (Nikon)
45x Nikon Plan Fluor Dry (NA 0.75) (Nikon)
60x Nikon Plan ApoChromat Oil (NA 1.4) VC (Nikon)
100x Nikon Plan ApoChromat Oil (NA 1.4) VC (Nikon)
Filter Blocks
Semrock DAPI BP 402/50 DM 409 BP 447/60
Semrock FITC BP 482/36 DM 506 BP 536/40
Semrock TRITC BP543/22 DM 562 BP 593/40
Camera
Nikon CCD Color Camera ‘Digital- Sight’Ds-Ri1
Full chip: 1280 X 1024 pixels
Physical pixel size: 6.44µm x 6.44µm pixel size (2/3 “chip)
Live display mode (DS-L2): 1280 x 1024 (max. 19 fps), 640 x 480 (max. 32 fps)
A/D conversion: 12-bits
CCD cooling device: Peltier Device, 10°C below ambient temperature (max.)
C-mount 1x
Software
NIS-Elements BR 4.13.05

Microscope body
Inverted DMi 6000 (Leica)
Motorized stage
Light sources
CoolLED pE 4000 (16 LED)
365/385/405/435nm
460/470/490/500nm
525/550/580/595nm
635/660/740/770nm
Lamp
Halogen Lamp for transmission imaging(Leica)
Objectives
5x HC PL FLUOTAR dry (NA: 0.15) (Leica)
10x HC PL APO dry (NA: 0.4) (Leica)
20x HC PL APO CORR CS multi-immersion (oil, glycerol, water) (NA: 0.7) (Leica)
40x HCX PL FLUOTAR dry (NA: 0.6) (Leica)
63x HCX PL APO oil immersion (NA: 1.4-0.6) (Leica)
Filter Blocks
A4 Filter Set : BP 340-380, DM 400 nm, BP 450-490 (Leica)
GFP Filter Set: BP 450-490, DM 500 nm, BP 500-500 (Leica)
ET-Y3 Filter Set: BP 530-560, DM 565 nm, BP 573-648 (Leica)
TRIPLE (Set 69008 Chroma):
BP 425-450 / DM 450-490
BP 495-510 / DM 510-560
BP 565-590 / DM 590-675
QUAD (Set 89401 Chroma):
BP 380-410 / DM 410-475
BP 475-500 / DM 500-545
BP 545-565 / DM 570-625
BP 625-650 / DM 660-735
External filter wheel – emission filter Blocks
Infinity 1 Lumenera (Color)
Full chip: 2048 x 1536 pixels
Physical pixel size: 3,2 µm
Frame rate: 12 frames/sec
Bit depth: 8 and 10 bits
C-mount: 0,63 X
HQ2 Photometrics
Physical Pixel Size : 6,45 x 6,45 µm
Full Chip : 1392 x 1040 pixels
C-mount: 1 X
Software
Metamorph v7.10.2.240

Microscope body
Upright Axio Imager.M2 (Zeiss)
Sources
Lamp
HXP 120 C _ 120 W metal halide lamp white source
HAL 100_Halogen lamp for transmission light ZEISS
Objectives
5x Fluar Dry (NA 0.25) (Zeiss)
20x Fluar Dry (NA 0.75) (Zeiss)
40x Achro Plan Water (NA 0.8) DIC (Deeping-Lens) (Zeiss)
40x Plan NeoFluar Oil (NA 1.3) DIC (Zeiss)
63x ApoChromat Oil (NA 1.4) DIC (Zeiss)
Filter Blocks
Number 49 DAPI G365 DM395 BP445/50 nm
Number 46 YFP BP500/20 DM 515 BP535/30 nm
Number 38 HE GFP BP 470/40 DM 495 BP525/50 nm
Number 43 HE DsRed BP550/25 DM 570 BP605/70 nm
Number 26 AF660 BP575-625 DM645 BP660-710 nm
Camera
AxioCam MRc (color) CCD Rev3 ZEISS (426508)
Full chip: 1388 X 1040 pixels = 1,4 Megapixels
Physical pixel size: 6.45 x 6.45 µm
Frame rate : 7 to 20 images/s
Digitization 12 bit / 18 Mhz pixel clock
Camera Mode : B/W RGB
One Stage Peltier Cooling
C-mount 60N-C 1”: 1 X (426114)
Modality
Widefield + Apotome 2
Software
AxioVision Rel. 4.8
Macroscopy

Microscope body
AZ100 MultiZoom upright macroscope (Nikon)
Light sources
130 W metal halide lamp Intensilight for fluorescence (Nikon)
Halogen lamp for transmission light (Nikon)
Objectives
AZ-Plan Apo 1X NA 0.1 WD 35 mm (Nikon) + DIC
AZ-Plan Fluor 2X NA 0.2 WD 45 mm (Nikon)
AZ-Plan Fluor 5X NA 0.5 WD 15 mm (Nikon) + DIC
Filter Blocks
A0 filter block: BP 340/26, DM LP380 nm, LP364 RS (Semrock)
DAPI filter block: BP 377/50, DM 409 nm, BP 447/60 (Semrock)
CFP-2432A filter block: BP 438/24, DM 458 nm, BP 483/32 (Semrock)
GFP-BP filter block: BP 472/30, DM 495 nm, BP 520/35 (Nikon)
GFP-LP filter block: BP 480/40, DM 505 nm, LP520 (Nikon)
YFP-2427A filter block: BP 500/24, DM 520 nm, BP 542/13 (Semrock)
TRITC-A filter block: BP 543/22, DM 562 nm, BP 593/40 (Semrock)
Camera
Color Ds-Ri camera, Nikon, (pixel size: 6.45 x 6.45 µm)
MultiZoom
Zoom 1X to 8X
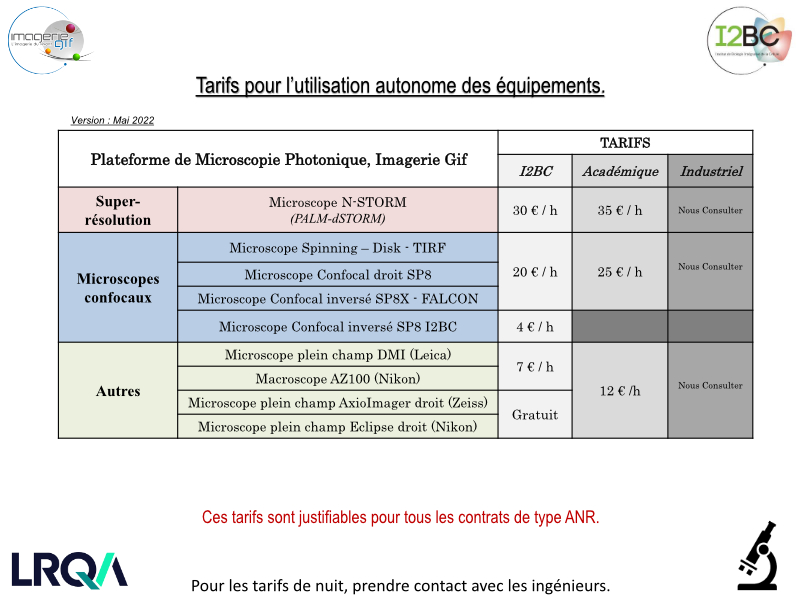
Rates
Training
Confocal microscopy workshop (5 days with alternating courses and practical work)
organised with CNRS Formation Entreprises.
Image analysis course on ImageJ / FIJI software (3 days).
Several sessions are organised every year with IFSEM and CNRS Formation entreprises.
Contact
Plateforme de Microscopie Photonique / Light Microscopy Facility
Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC)
1 avenue de la Terrasse
91198, Gif-sur-Yvette, France.
Le Bars Romain, Head of facility, Research Engineer
Mail : romain.lebars@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 40
Lecart Sandrine, Research Engineer
Mail : sandrine.lecart@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 40
Valérie Nicolas, Research Engineer
Mail : valerie.nicolas@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 40
Amandine Dumazel, Engineer
Mail: amandine.dumazel@i2bc.paris-saclay.fr
Phone: +33 1 69 82 46 40
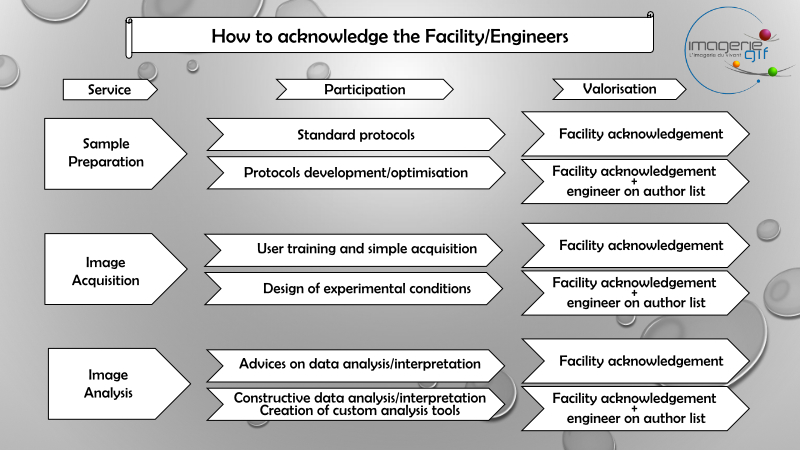
Acknowledgements
Funding is essential for the proper functioning and development of the Imagerie-Gif facilities. To this end, it is essential to acknowledge the facility as soon as you have benefited from the help of the engineers or any equipment.
Use this sentence to acknowledge the Light Microscopy Core Facility :
The present work has benefited from Imagerie‐Gif core facility supported by l’Agence Nationale de la Recherche (ANR-10-INBS-04/FranceBioImaging ; ANR‐11‐IDEX‐0003‐02/ Saclay Plant Sciences )
Use this address for the Light Microscopy Core Facility :
Light Microscopy Facility, Imagerie-Gif, Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC), 91198, Gif-sur-Yvette, France.