Lipid trafficking and membrane contact sites
Topics
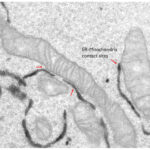
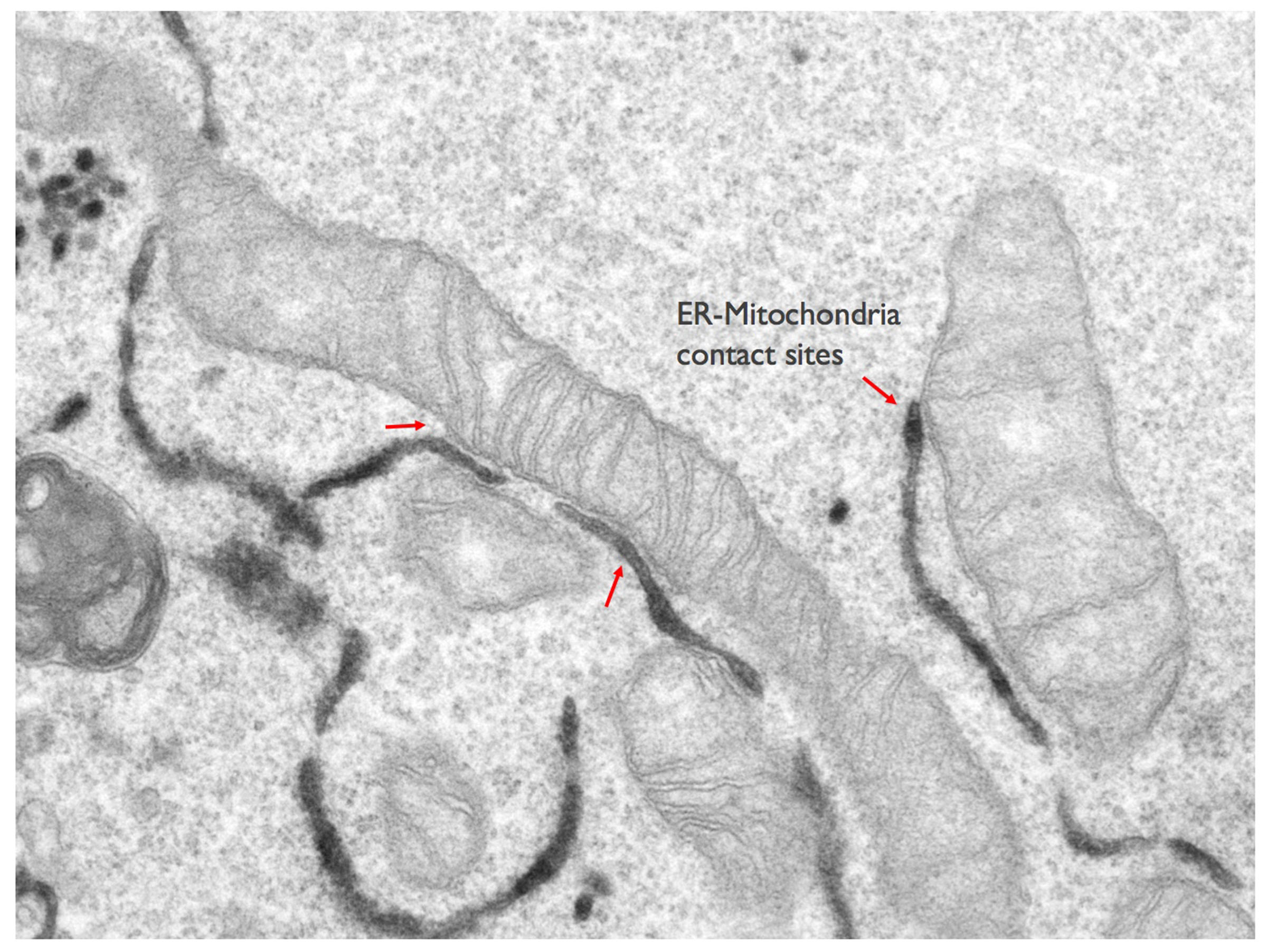
The molecular dissection of the protein components of ER-mitochondria contact sites is the first step towards understanding not only how they are formed, but also to elucidate their physiologically conserved roles. Some of the components of ER-mitochondria contacts have started to be identified in recent years and the list is rapidly growing. Yet, the identity of ER-mitochondria contact sites components mediating lipid trafficking is still largely uncovered. We use a combination of various biochemical (proteins and organelles purification, Mass spectrometry,..) and imaging (including confocal microscopy, immuno-electron microscopy and 3D electron microscopy) approaches, as well as in situ and in vitro functional assays (mitochondria respiratory activity, calcium imaging, lipid transfer assays,..) to identify novel components of ER-mitochondria contact sites involved in lipid transfer, to analyse their localisation within these tiny structures and to study how they work all together to regulate mitochondria lipid composition, morphology and homeostasis.
Once considered to be static, isolated organelles, mitochondria are now viewed as highly mobile entities that form dynamic networks within cells. The mitochondrial network constantly remodels and adapts to carry out their multiple functions, through changes in mitochondrial morphology, number and distribution. Mitochondrial dynamics is a highly regulated process that directly depends on fission and fusion events as well as on the transport along the cytoskeleton within the cell. Changes in mitochondrial dynamics have been associated with a broad array of neurodegenerative diseases, including Parkinson’s, Alzheimer’s, Hungtington’s and Charcot-Marie-Tooth diseases. ER-mitochondria contact sites have been shown to regulate multiple aspects of mitochondrial dynamics, but very little is still known about the underlying molecular mechanisms. This project aims at elucidating the molecular mechanisms by which ER-mitochondria contacts regulate mitochondrial dynamics (fusion, fission, transport) and how these processes are regulated by lipids, and functionally coupled to the lipid transport machinery at specific ER-mitochondria contact site subdomains. To this end, we are using a combination of in situ biochemical assays and imaging approaches including live-cell imaging of mitochondria (Movie: Mitochondria in fission) and electron microscopy.
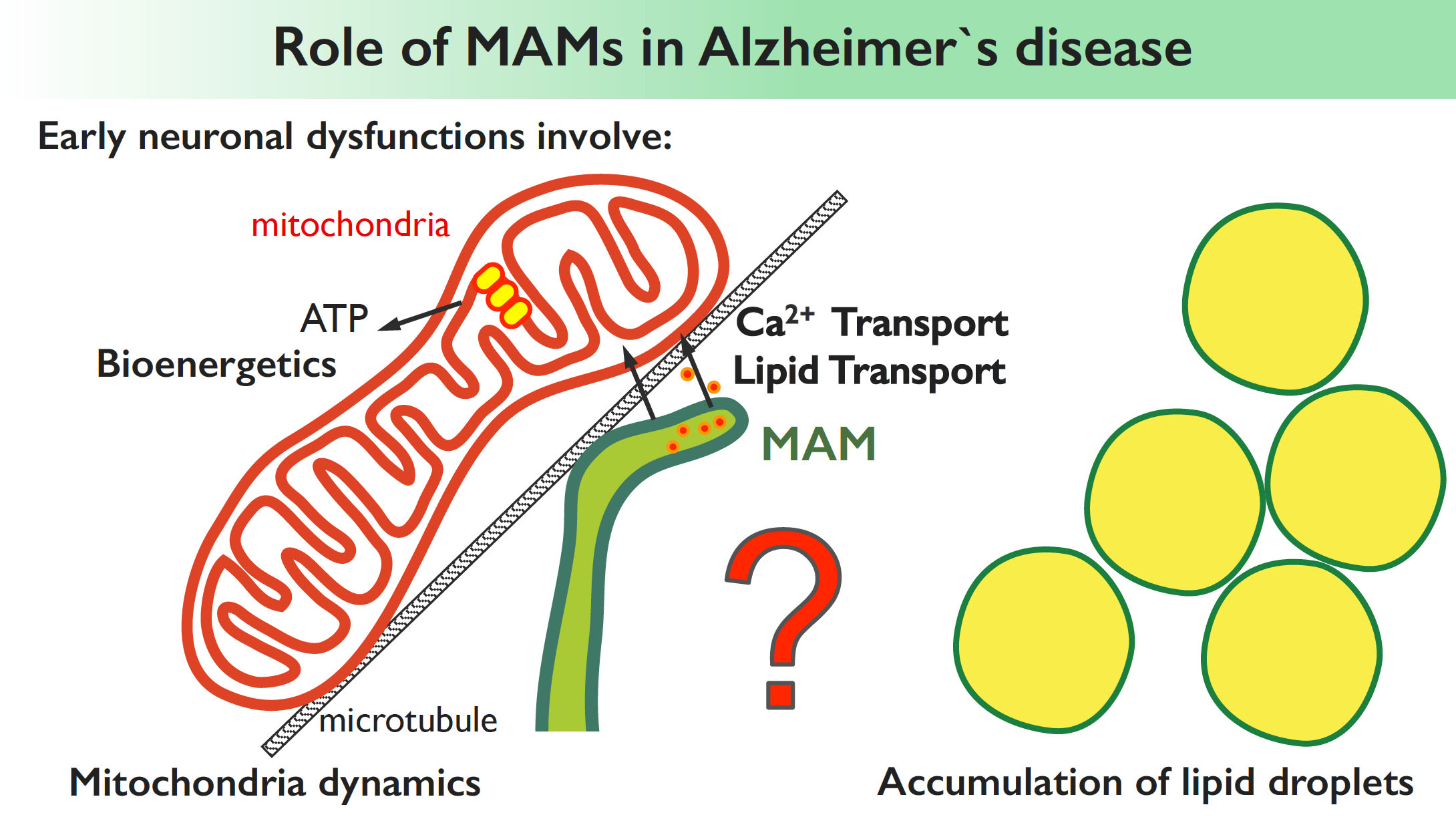
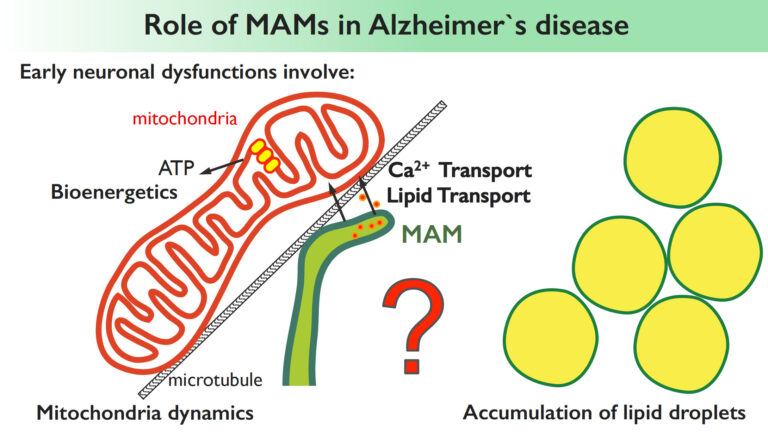
Increasing evidence suggests a functional link between the MAMs, an ER subdomain in contact with mitochondria, and neurodegenerative disorders such as Alzheimer’s disease (AD). However, the role of MAM in AD pathogenesis is still mysterious. The accumulation of extracellular amyloid plaques and neurofibrillary tangles are known as the major pathological hallmarks of Alzheimer’s. However, other neuronal dysfunctions, often occurring years before the appearance of the plaques, manifest at early preclinical stages of AD, and involve mitochondria [altered lipid and calcium trafficking to mitochondria, altered mitochondrial dynamics and bioenergetics and lipid droplet accumulation]. Yet, the causal mechanisms of these apparently unrelated features remain unknown. We hypothesize that the MAMs could be the central regulator of all these processes and, by studying the role of MAMs in physiological and in AD settings we aim to provide a first molecular and functional link of all these apparently unrelated features of Alzheimer’s disease. We also aim to identify novel molecular components of MAMs altered in Alzheimer’s and other neurodegenerative disease contexts.