Lipid trafficking and membrane contact sites

We study how organelles communicate via membrane contact sites, with emphasis in non-vesicular lipid trafficking and contacts involving the endoplasmic reticulum and the mitochondria.
Overview
One of the hallmarks of eukaryotic cells is the presence of membrane-enclosed organelles that allow segregation of specific biochemical processes. However, membrane compartmentalisation also creates the need for organelles to communicate with each other to coordinate their activities. Our lab studies how organelles communicate via membrane contact sites and we focus on those involving the endoplasmic reticulum (ER) and the mitochondria. We use a combination of in-situ imaging, biochemical and cell-free approaches to study how information material and small metabolites (i.e. lipids) are exchanged at membrane contact sites, and how these transport activities impact on organelles morphologies, functions and dynamics.
Characterization of ER-mitochondria contact sites
involved in non-vesicular lipid transport
Background
The defining feature of eukaryotic cells is the segregation of specific functions in different membrane-enclosed compartments called organelles. Although cell compartmentalization has obvious advantages, it also imposes the need for the various organelles to communicate via transport routes that allow exchange of metabolites and information across the cell. Each organelle has a unique lipid composition that is essential to preserve its distinct structural and functional identity among the other organelles, and requires a tight regulation of lipid metabolism and transport pathways. Disruption of lipid trafficking and metabolism leads to human pathologies such as obesity, diabetes, cancer and neurodegenerative diseases.
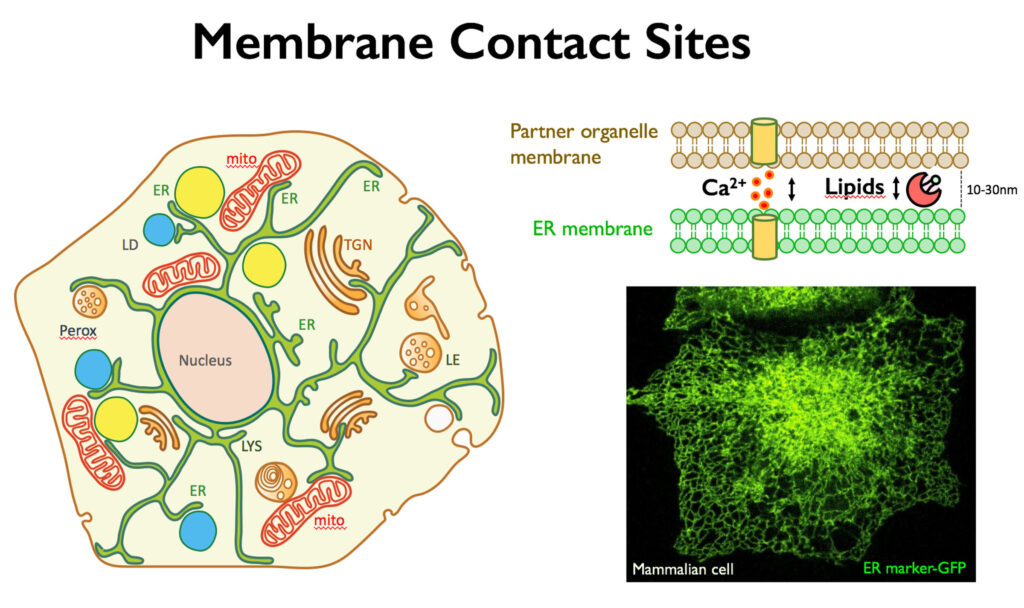
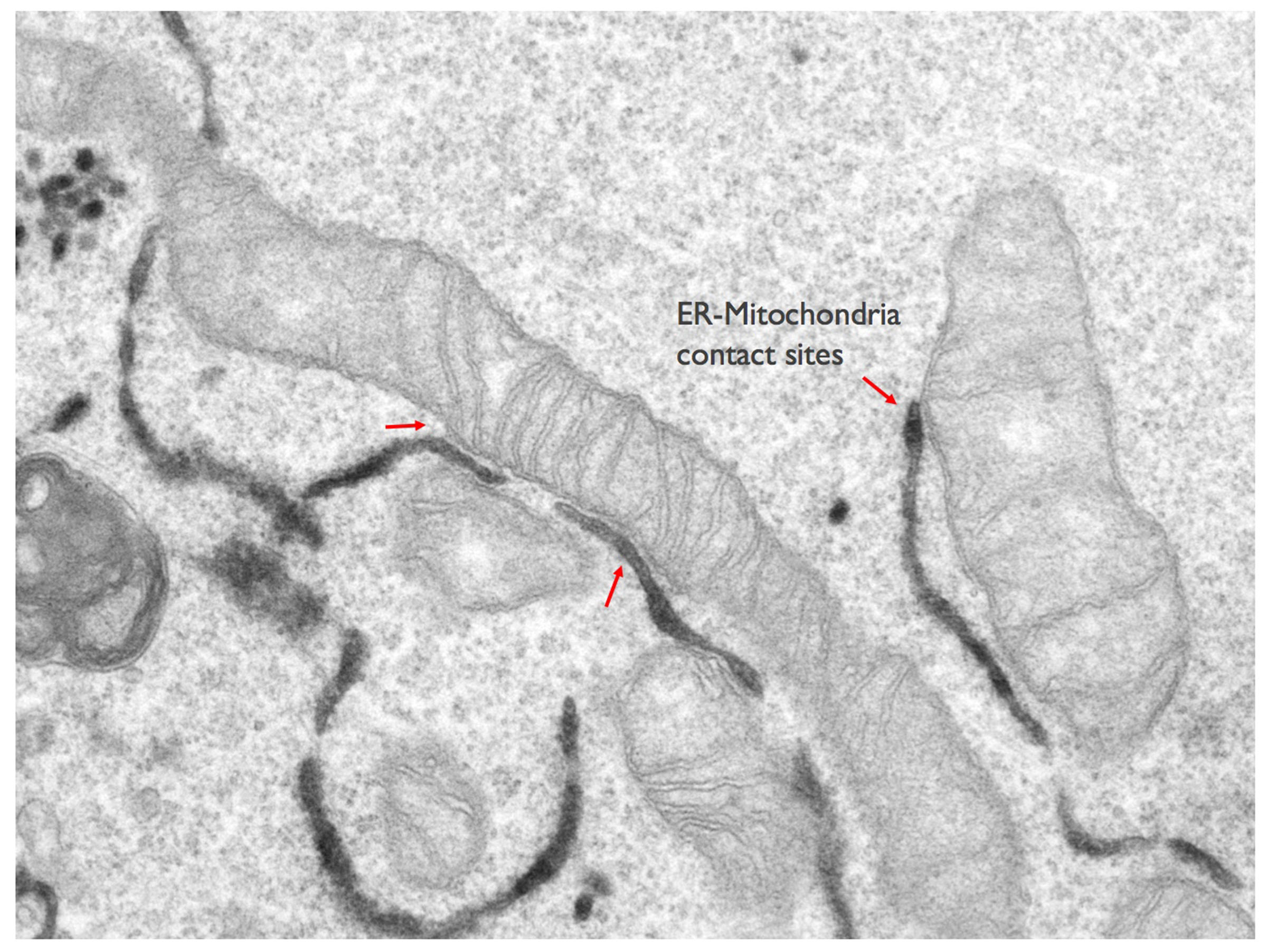
For a long time vesicle-mediated transport of proteins and lipids has been thought to be the main mechanism for interorganelle communication. The last decade has radically changed this view, and membrane contact sites have emerged as primary highly regulated transport routes for interorganelle communication, essential for cellular homeostasis. Membrane contact sites are regions of close apposition between organelles that facilitate direct exchange of small metabolites (i.e. lipids and calcium) across the two partner membranes in a vesicle-independent way. The endoplasmic reticulum (ER), major site of lipid synthesis and calcium store in eukaryotic cells, forms a dynamic membrane network that spreads throughout the cell and is engaged in membrane contact sites with nearly every other organelle, including mitochondria, lipid droplets and plasma membrane.
Non-vesicular lipid transport at membrane contact sites is extremely important in the case of organelles, such as the mitochondria (or the lipid droplets), that are largely excluded by the classical vesicular transport pathways. However, the mechanism underlying these transport processes and proteins involved are still largely unknown.
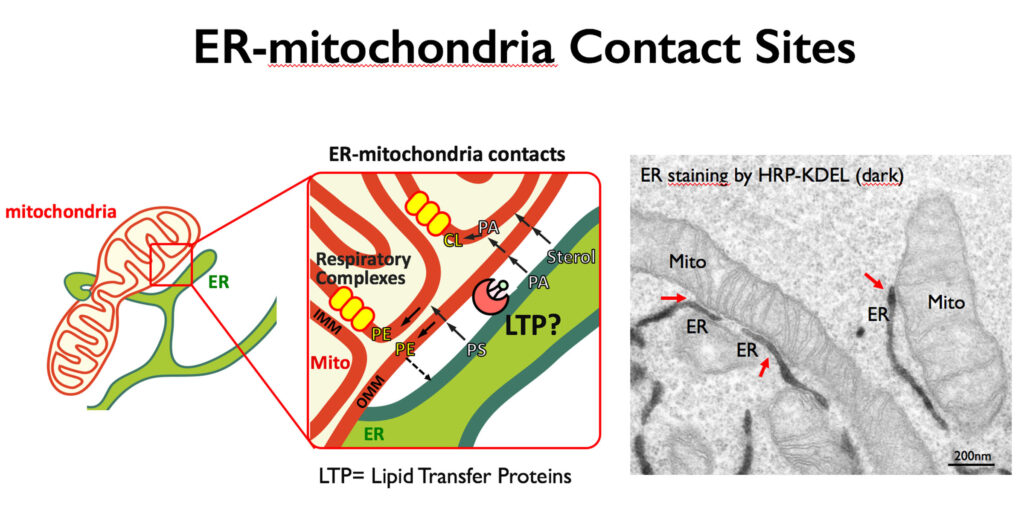
Our team is dedicated to uncovering the molecular mechanism regulating lipid trafficking at membrane contact sites and how these transport processes are integrated within other cellular communication pathways in physiological and pathological settings. In particular we are interested in understanding how lipids are exchanged at ER-mitochondria membrane contact sites, and how these transport activities impact on mitochondria morphology, function and dynamics. (Figure: Lipid transport at ER-mitochondria contacts).
Mitochondria are dynamic organelles that participate in a plethora of biological processes including energy conversion, metabolism, signaling and apoptosis, and are therefore of utmost importance for cell viability. To fulfill their multiple tasks, mitochondria adopt a variety of morphologies resulting from a constant reshaping by fusion and fission events. This dynamic behavior imposes the necessity to maintain a defined protein and lipid composition of both outer and inner mitochondria membranes in order to preserve the functional integrity of mitochondria and the spatial organization of complex biochemical reactions. Since mitochondria do not have the enzymes to synthesize all their lipids and lipid precursors, they require a continuous and coordinated supply of membrane lipids from juxtaposed ER subdomains, also called MAMs (Mitochondria Associated -ER- Membranes), to carry out their physiological processes and maintain their membrane integrity (Figure: Lipid transport at ER-mitochondria contacts. However, the underlying mechanisms and the proteins involved in these transfer processes are still largely unknown.
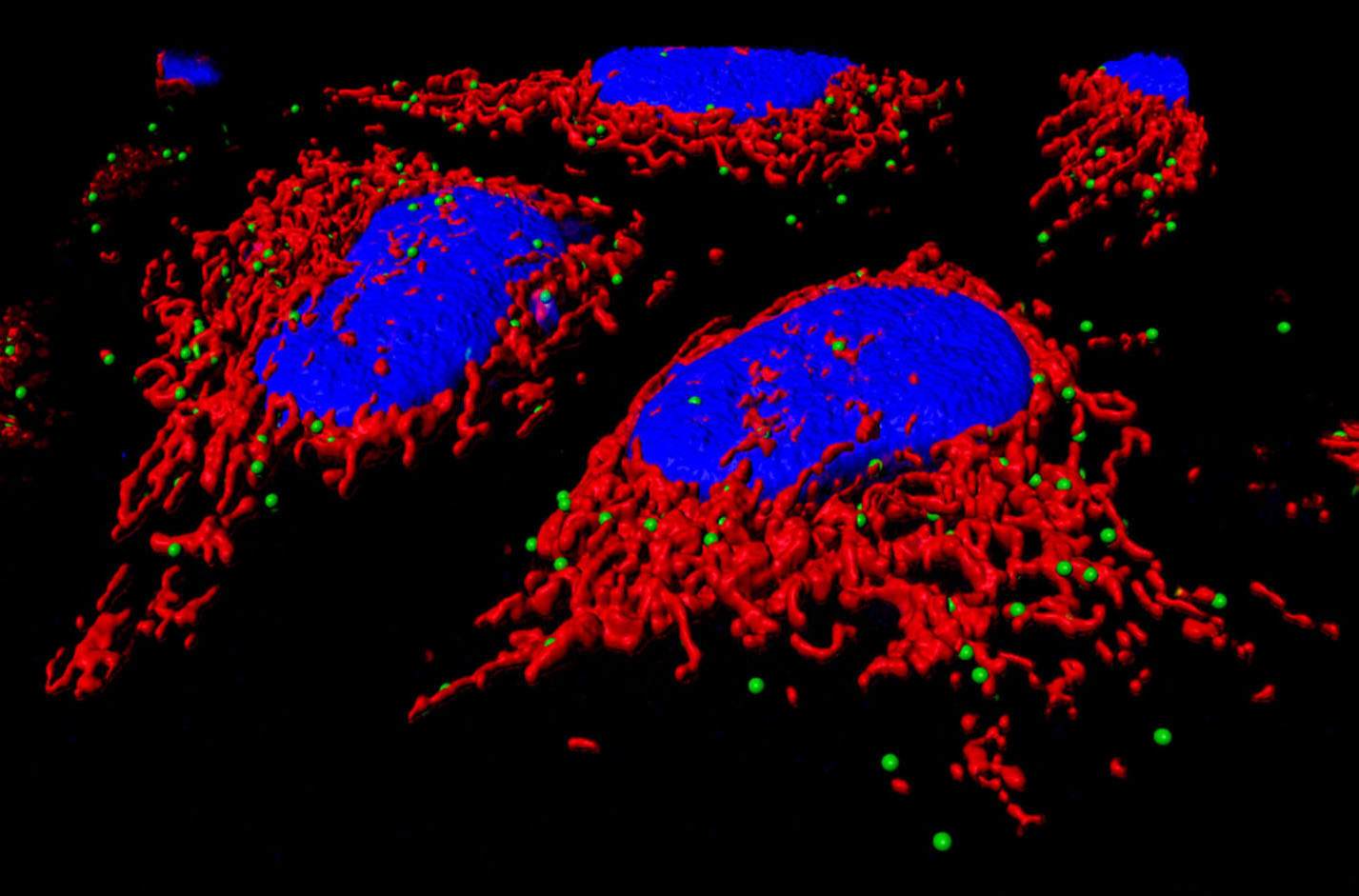
By combining multiple biochemical and imaging approaches, we have recently found that the lipid transfer proteins (LTPs) ORP5 and ORP8 [members of the Oxysterol-binding protein (OSBP)-related protein (ORP) family] are novel components of ER-mitochondria contact sites, in addition to ER-PM contacts (Figure: ORPs and imaging approaches of the team). We have also shown that these proteins are required for mitochondria morphology and respiratory function and we hypothesize that their primary function is to mediate lipid transport (i.e. Phosphatidylserine, PS) at ER-mitochondria contact sites. Studying the role of these proteins and of their binding partners offers the unique opportunity to functionally characterize ER-mitochondria contact sites involved in lipid transport.
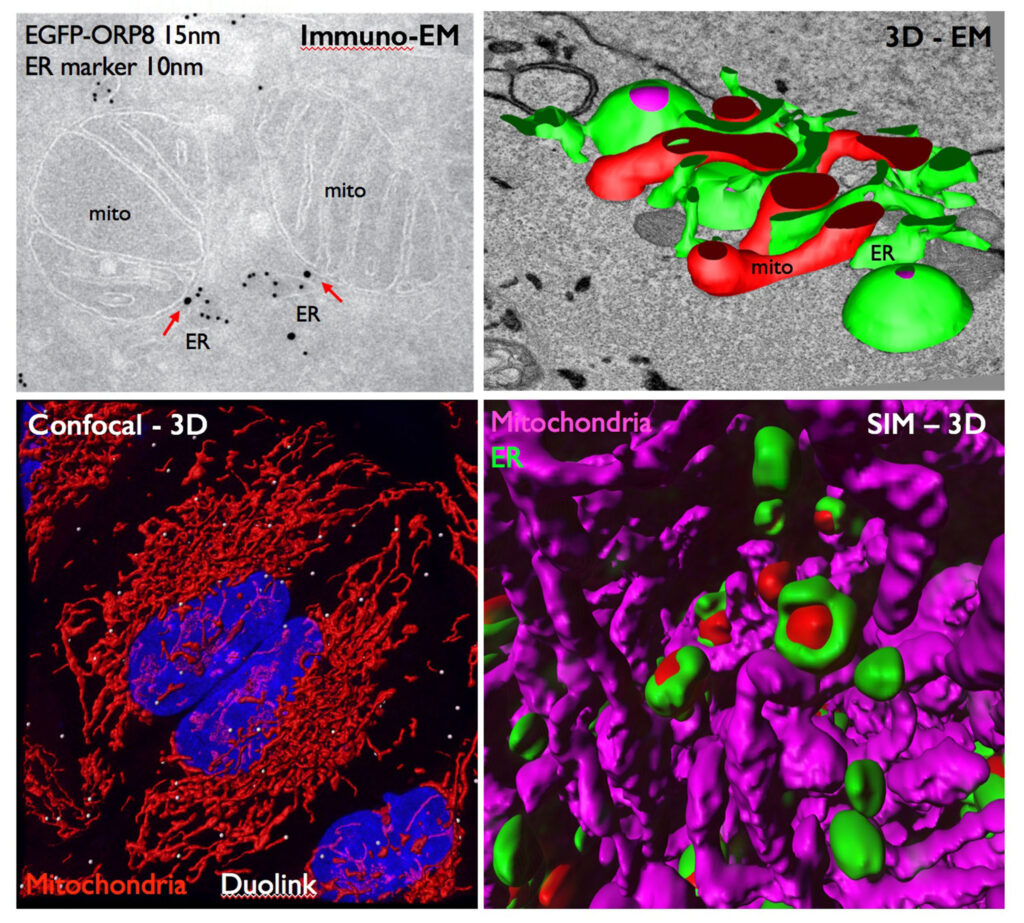
By using a combination of imaging and morphological approaches including 3D electron microscopy (EM), biochemical and subcellular fractionation techniques (Figure: ORPs and imaging approaches of the team), as well as cell-free lipid transport assays we aim to:
- Biochemically, morphologically and functionally characterize ER-mitochondria contact sites involved in lipid transport.
- Study the impact of lipid transport at ER-mitochondria contact sites on mitochondria morphology, activity and dynamics.
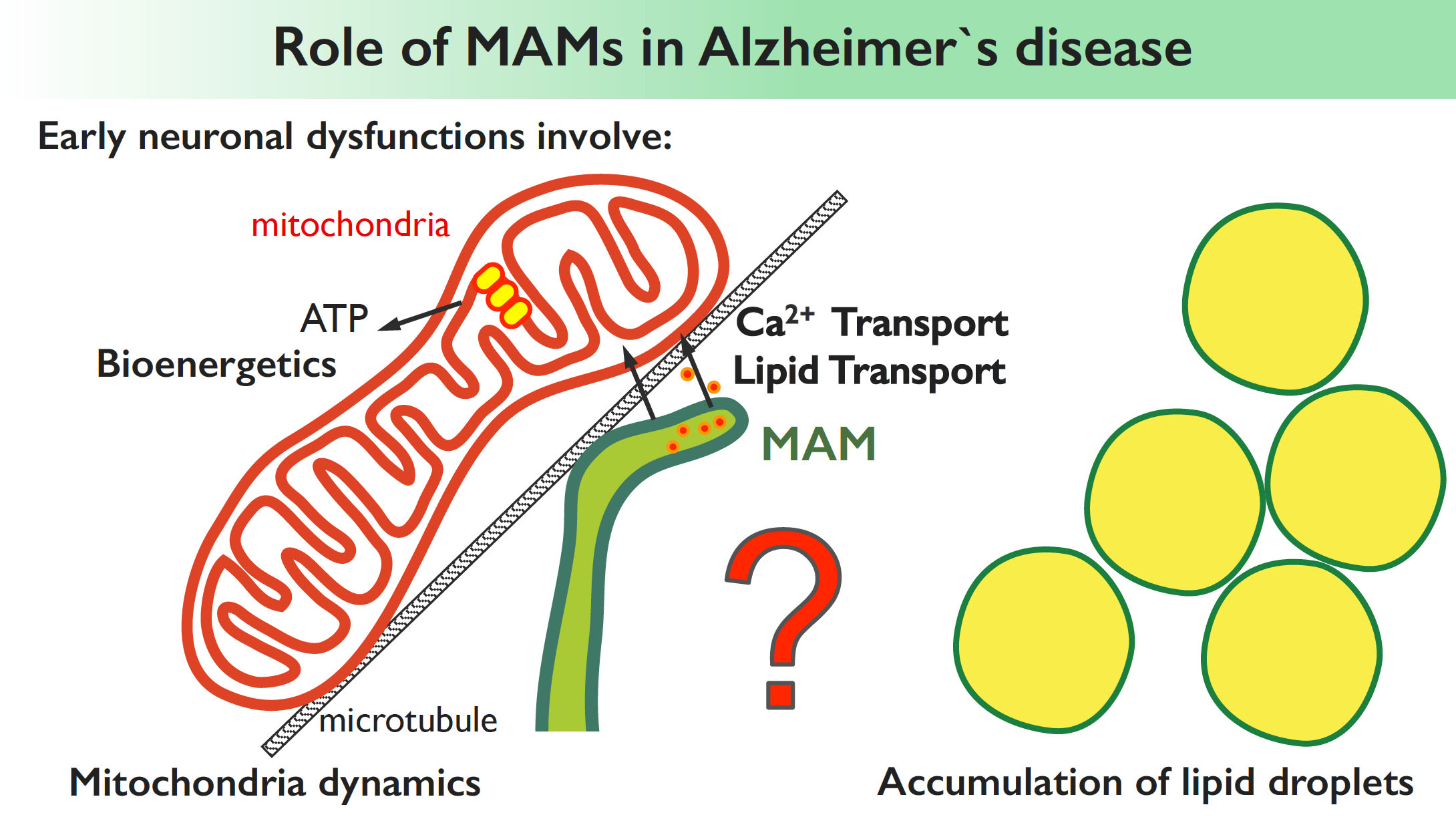
- Study how lipid transport activities at MAMs are coordinated with intracellular lipid trafficking pathways at other contact sites in physiological and pathological (i.e. Alzheimer’s disease) settings.
Understanding the functions of ER-mitochondria contact sites is not just important for comprehending fundamental physiological processes but also for understanding pathogenic processes in various diseases (such as neurodegenerative disorders, i.e. Alzheimer’s) induced by disruption of these contact sites
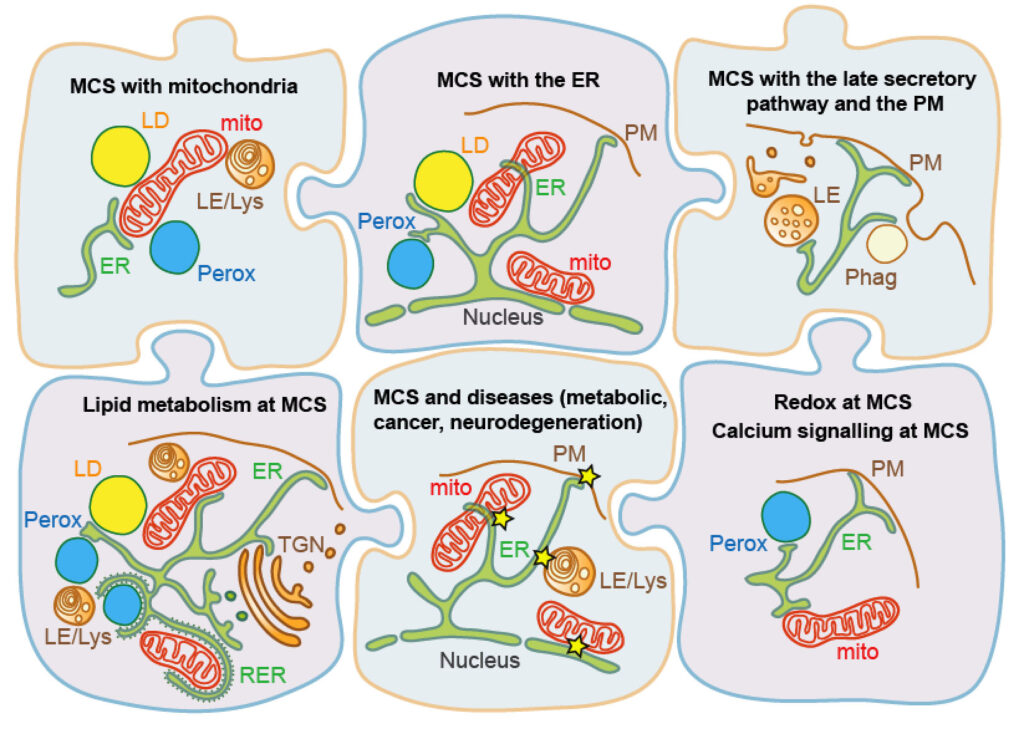
Topics
team

Group Leader Senior Researcher

Researcher
Engineer assistant
Engineer assistant

PhD student
PhD student
Master Student
team
Vera CARDOSO
Post Doc
Valentin GUYARD
PhD student
Naima EL KHALLOUKI
Engineer CNRS
Gaelle DUFAYET-CHAFFAUD
Engineer Inserm
Esther Aurora GIL HERNANDEZ
M2 student
Clémence MARRE
L3 student
Lab life...
team pictures Jan 2021
team pictures Jan 2021
team pictures Jan 2021
Lunch meeting in Gif sur Yvette gardens Spring 2020
Giordano-Legouis’s teams picture 2020
Giordano’s team picture May 2019
Cell culture fun 2019
Meeting with Miochondria at the EM microscope 2019
Latest publications
For all the publications of the Team click on the button below.
External funding

Foundation Vaincre Alzheimer (FVA)
(2020-2022)

Foundation Schlumberger for the Education and Research (FSER) (2020-2022)

ATIP-Avenir
(2017-2022)

ANR Jeune Chercheur
(2014-2017)

Marie Curie CIG
(2014-2018)
Collaborations
National collaborators
Dr. Rachid Thiam, Physics Department of ENS, Paris, France.
Prof. Thierry Galli, Institute of Psychiatry and Neuroscience of Paris (IPNP), France
Dr. David Tareste, Institute of Psychiatry and Neuroscience of Paris (IPNP), France
Dr. Etienne Morel, Institute Necker Enfant-Malades (INEM), France
International collaborators
Prof. Vesa Olkonnen, Director of Minerva Foundation Institute for Medical Research, Biomedicum 2U, Helsinki, Finland
Prof. Patrizia Agostinis, KU Leuven, Belgium
Prof. Johan Swinnen, KU Leuven, Belgium
Prof. Wim Annaert, VIB-KU Leuven, Belgium