Rhabdovirus
Topics
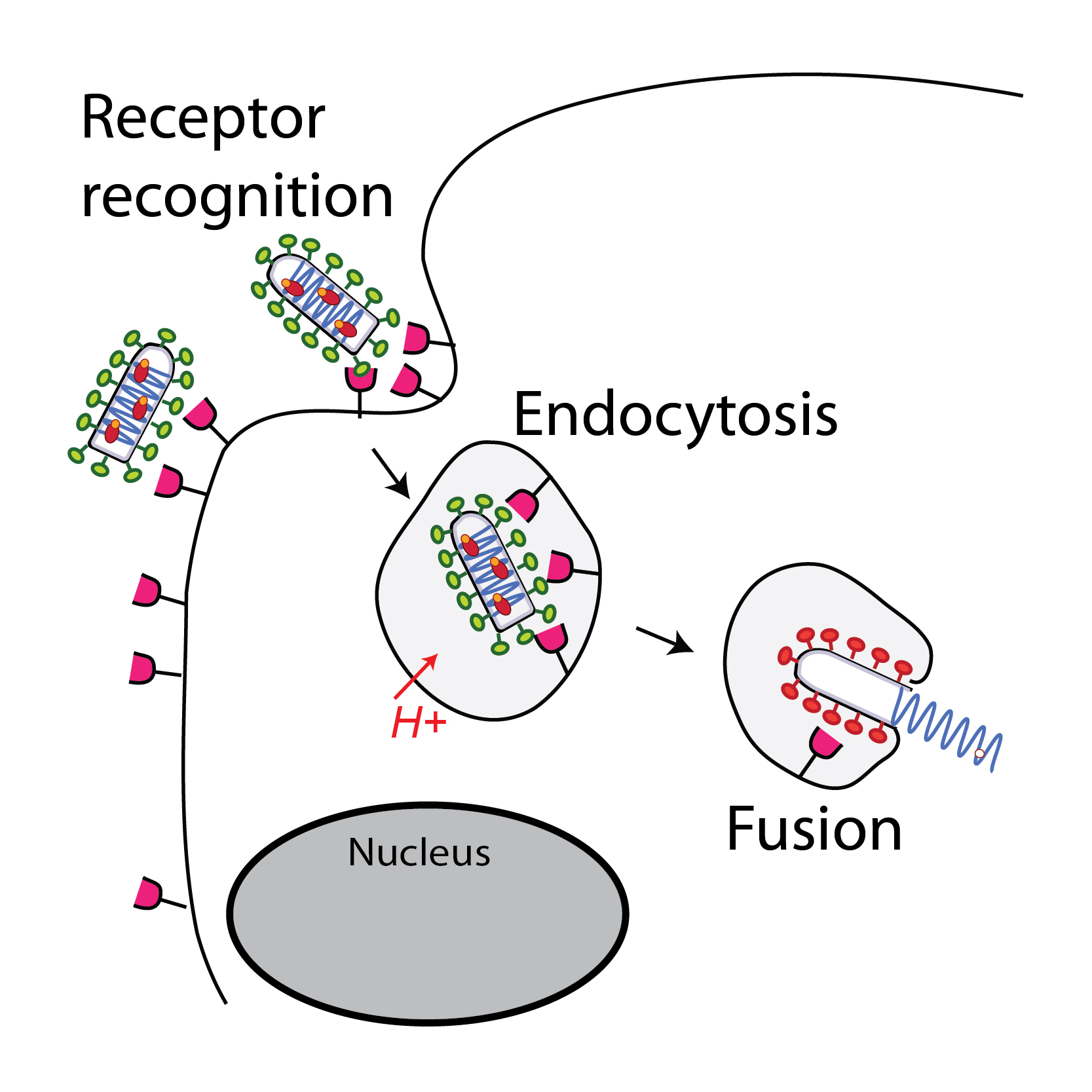
Glycoprotein G plays an essential role in the early stages of infection. First, by interacting with a cellular receptor, it enables viral attachment. Then, after endocytosis of the virion, G undergoes a major conformational change, induced by the acidification of the inner of the endosome, which catalyzes the fusion of the viral and endosomal membranes.
We seek to elucidate the mechanism of membrane fusion. For this, we have developed functional tests to characterize the fusion properties of G and biochemical techniques to follow its structural transition.
We also use X-ray crystallography and cryo-electron microscopy to determine the structure of glycoproteins of rhabdoviruses in their different conformations alone or in complex with partners. We have thus determined the initial, final and even intermediate conformations of the G of several rhabdoviruses, as well as the structure of the VSV glycoprotein in complex with two CR domains (cystein-rich) of its natural receptor: the LDL receptor (LDL -R).
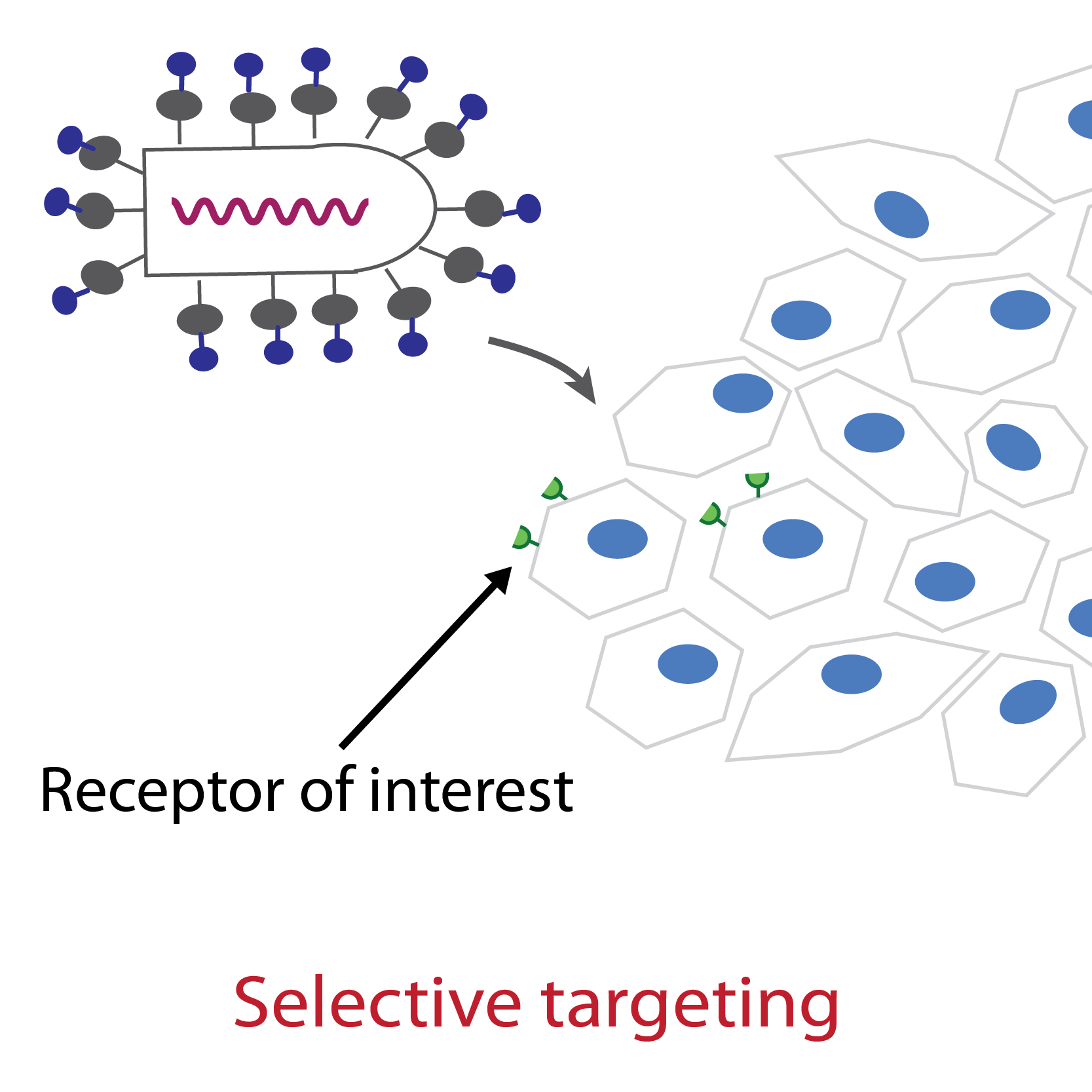
The structure of the complex between VSV G and its receptor as well as our mutagenesis work have shown that VSV G has evolved to recognize specifically the CR domains which are present in the receptors that are members of the LDL-R family. The members of the LDL-R family therefore constitute the major receptors for VSV (or even the only ones), including in insect cells. We have identified G residues whose mutation abolishes its ability to bind its natural receptors without however abolishing its fusion properties. This paves the way for the specific targeting of G to receptors of interest (e.g. tumor antigens) and opens up prospects for gene or oncolytic therapy.
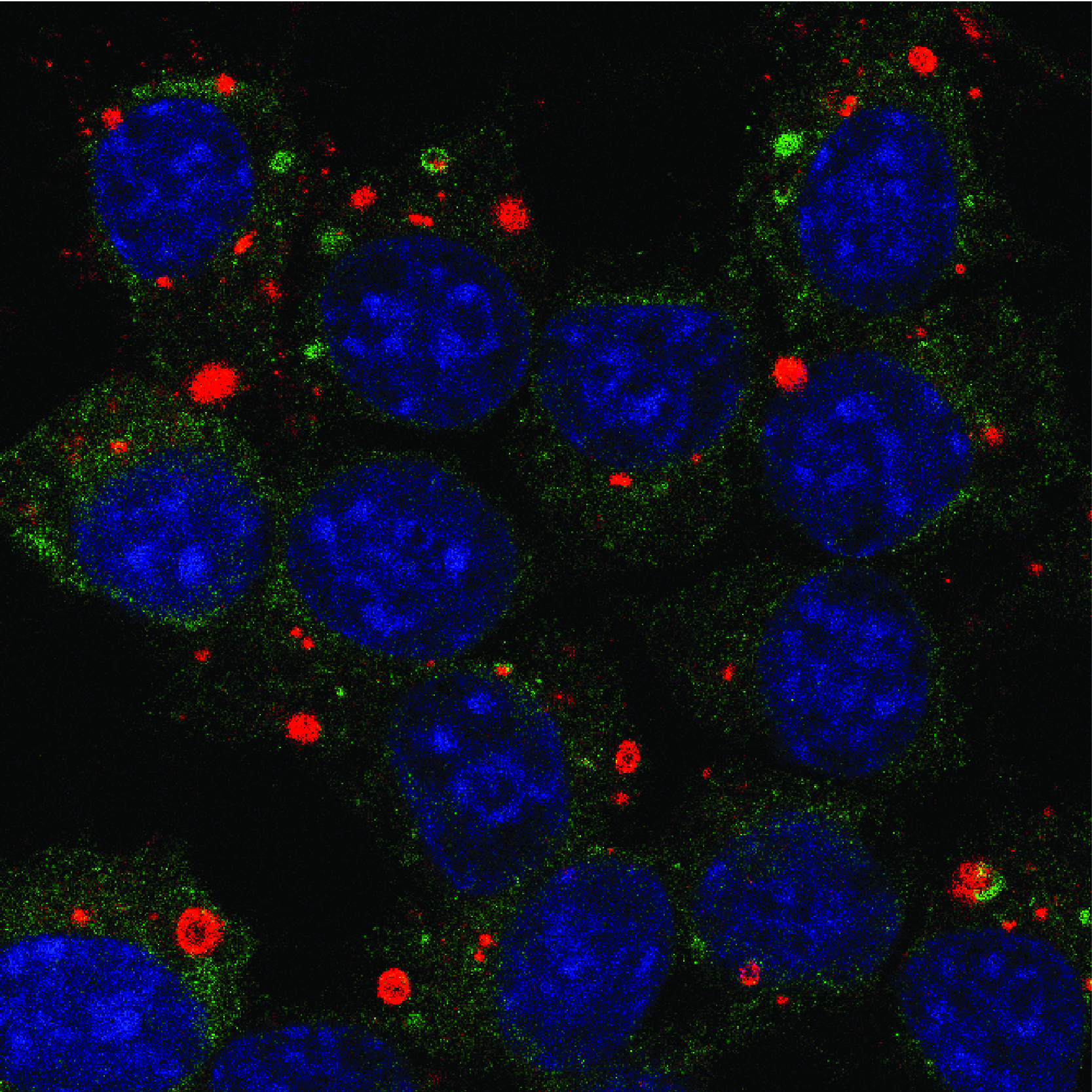
In the cytoplasm of cells infected by rabies virus, there is the formation of spherical inclusion bodies called Negri bodies. These structures constitute active sites for the synthesis of viral RNAs and are in fact true viral factories. Recently, we have shown that Negri bodies are membrane-less organelles formed by the separation of liquid phases. They fuse together, reversibly deform when they encounter a physical barrier, and disappear when the cell is subjected to hypotonic shock. This observation has been extended to many other viruses belonging to the order Mononegavirales (measles virus, Ebola virus, respiratory syncytial virus, etc.). This discovery is a paradigm shift in the understanding of the interactions between these viruses and their host cell and it opens up new research themes at the interface between biology and physics.
One of the first cellular responses to infection is the production of interferon (IFN), which will activate signaling pathways and transcription of cellular genes, inducing an antiviral state. Viruses have evolved to counteract the work of IFN. Thus, the P protein of the rabies virus (i) inhibits the production of IFN by preventing IRF-3 factor phosphorylation, (ii) inhibits IFN signaling by blocking the nuclear translocation of the transcription factor STAT1 and the binding of this factor to DNA and finally (iii) interacts with the PML protein (induced by IFN) and blocks its antiviral activity. The P protein is therefore a multifunctional IFN antagonist that targets several innate immunity processes in different cell compartments. We are seeking to better understand the molecular mechanisms behind these P activities, but also to characterize the interactions between the viral factory and the cellular players in the innate immune response.