Biochemistry of Metalloproteins
and Associated Diseases
Transition metals are essential elements of life as the main constituents of the active sites of metallo-enzymes involved in a multitude of key biological processes
such as respiration, energy production, catalysis, signaling and genome protection.
Overview
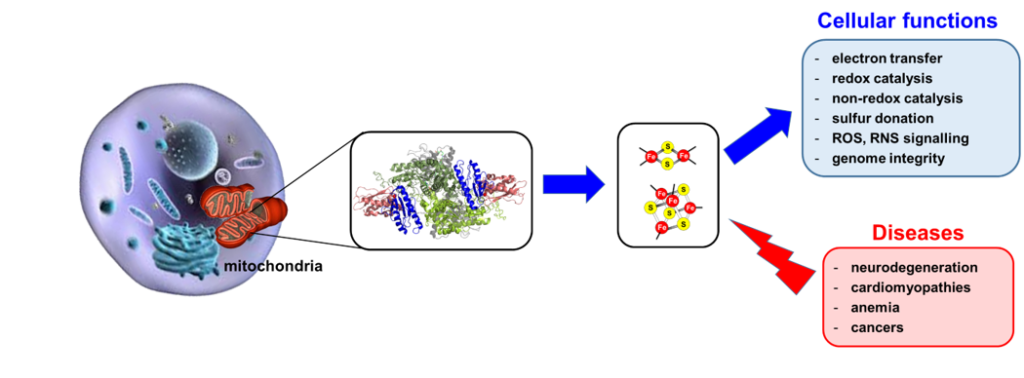
Transition metals are the main constituents of the active sites of several enzymes and proteins where they provide redox, catalytic and structural properties. The most frequently used transition metals in biological systems are iron, manganese, copper, nickel, cobalt, molybdenum and zinc. Among them, iron is one of the most abundant. It is used in the form of heme iron centers, non-heme iron centers and iron-sulfur (Fe-S) clusters. The BMAD team is focusing its research on metallo-proteins, essentially Fe-S clusters: their biosynthesis, their roles in biology, the connections with iron and sulfur metabolisms and the diseases linked to the defects in the synthesis of these metallo-cofactors.
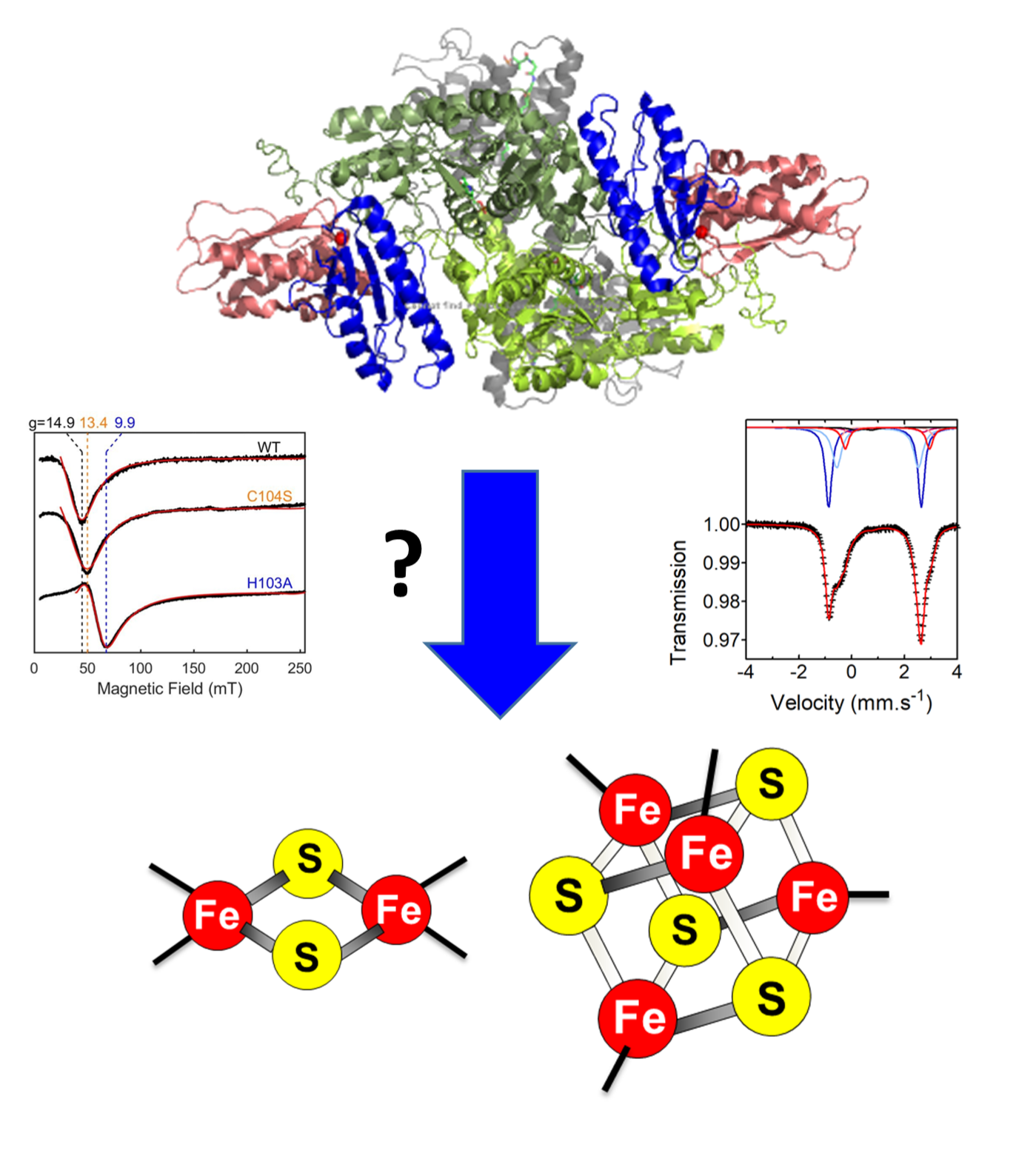
Fe-S clusters are small prosthetic groups of protein made of iron and sulfide ions. The most common forms in biological systems are the [2Fe2S] and [4Fe4S] clusters. Owing to their broad redox properties and their abilities to bind and react with small molecules and substrates, Fe-S cluster enzymes and proteins fulfill a wide range of biological functions: electron transfer for the production of ATP, redox and non-redox catalysis, signaling and maintenance of genome integrity. They are ubiquitous in living organisms (eukaryotes, bacteria and archaea), even some viruses use Fe-S clusters for their replication. Fe-S clusters are overly reactive and too labile to be obtained from food, they must be biosynthesized de novo in each cell by multi-protein machineries. Mitochondria is the primary site for the biosynthesis of [2Fe2S] clusters that are the building blocks for the assembly of [4Fe4S] clusters which occurs in both mitochondria and the cytosol. These processes are achieved in two main steps: assembly onto scaffold proteins and transfer/insertion into recipients enzymes. The mechanisms of these processes are complex and still unclear. Many diseases are caused by the defects in their biosynthesis or the dysfunction of a specific Fe-S enzyme, but the mechanisms leading to these diseases are still poorly understood. The lack of clear description of this fundamental process and the high medical importance of Fe-S clusters has spurred intensive research to understand how these cofactors are biosynthesized and assembled into proteins, which is one of the focus of the BMAD team.
Using a combination of cellular, biochemical, spectroscopic and structural approaches, the BMAD team is aiming to:
-Unravel the mechanism of Fe-S clusters biosynthesis in eukaryotic and prokaryotic organisms (Research Theme N°1)
-Understand the integration of the Fe-S clusters biogenesis pathways into the cellular context of iron and sulfur metabolisms (Research Theme N°1)
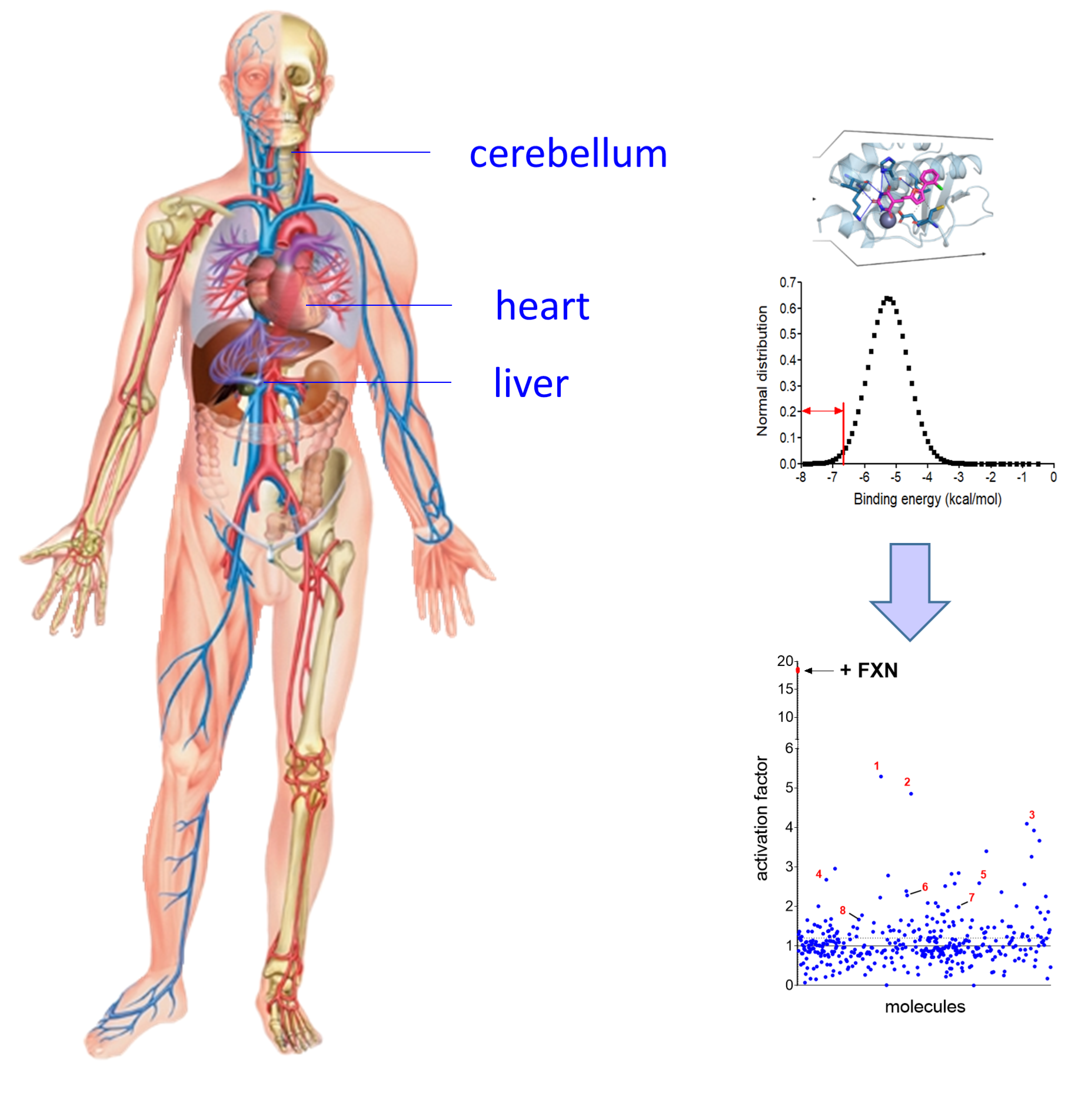
-Develop strategies to discover drug candidates for the treatment of the diseases caused by defects in Fe-S cluster biosynthesis and in specific Fe-S cluster enzymes. We are mainly studying Friedreich’s ataxia, a neurodegenerative and cardiac disease (Research Theme N°2).
-Elucidate the functional roles of newly discovered Fe-S clusters in enzymes and proteins (Research Theme N°3)
Experimental approaches
- Reconstitutions of metallo-proteins and cellular machineries in vitro using purified proteins
- Manipulation under anaerobic conditions to prevent metal and sulfur oxidations
- Cellular approaches, genetics in the yeast model, site-directed mutagenesis
- Biochemical assays: metal quantifications, detection of reactive sulfur species and cysteine modifications (persulfides, disulfides, sulfenic acids…), isotopic labelling
- Kinetics, modelling to assess enzyme activities
- Spectroscopic techniques to probe metal centers: UV-visible, CD, EPR, NMR, EXAFS, XANES, Mössbauer
- Protein structure determination: NMR, cryo-EM, crystallography, modelling and prediction
- High throughput screening assays of chemical libraries
- Drug testing in animal models
Highlights
Research Themes
team

Group Leader Senior Researcher

Researcher
Master Student

PhD student
team

Benoit D'AUTRÉAUX
Group Leader
Research Director
INSERM
Hubert GORNY

Post-Doc FARA
Kristian WANT

PhD CEA
Rémi MOR-GAUTIER

PhD ANR
Latest publications
- Gervason, Sylvain, Rafal Dutkiewicz, Kristian Want, Rania Benazza, Rémi Mor-Gautier, Aneta Grabinska-Rogala, Christina Sizun, et al. . “The ISC Machinery Assembles [2Fe-2S] Clusters by Formation and Fusion of [1Fe-1S] Precursors.” Nature Chemical Biology, (2025) January. https://doi.org/10.1038/s41589-024-01818-8.
- Want, Kristian, and Benoit D’Autréaux. “Mechanism of Mitochondrial [2Fe-2S] Cluster Biosynthesis.” Biochimica Et Biophysica Acta. Molecular Cell Research (2024) 1871 (8): 119811. https://doi.org/10.1016/j.bbamcr.2024.119811.
- Srour B., Gervason S., Want K., Monfort B., Sizun C., Schunemann V., Fonda E., Dutkiewicz R., Goldberg P., Guigliarelli B., Burlat B., D’Autréaux B.* Iron insertion at the assembly site of the ISCU scaffold protein is a conserved process initiating Fe-S cluster biosynthesis J. Am. Chem. Soc. (2022), 144(38), 17496-17515
- Monfort B., Want K., Gervason S., D’Autréaux B.* Recent advances in the elucidation of Frataxin biochemical function open novel perspectives for the treatment of Friedreich’s ataxia Front. Neurosci. (2022), 16, 1-17
- Gervason S., Srour B., D’Autréaux B.* A Fast and Ratiometric Method for Quantification of Cysteine-Bound Persulfides Based on Alkylation Gel-Shift Assays Methods Mol. Biol. (2021), 2353, 191-205
- Maková B., Mik V., Lišková B., D’Autréaux B., Hajdúch M., Strnad M., Voller J. Cytoprotective activities of kinetin purine isosteres Bioorg. Med. Chem. (2021), 33, 115993
- Gervason S., Srour B., D’Autréaux B.* Mechanism of iron-sulfur cluster assembly: in the intimacy of iron and sulfur encounter Inorganics Review (2020), 8(10), 55-93
- Russi M., Martin E., D’Autréaux B., Tixier L., Tricoire H., Monnier V. A Drosophila model of Friedreich Ataxia with CRISPR/Cas9 insertion of GAA repeats in the frataxin gene reveals in vivo protection by N-Acetyl Cysteine Hum. Mol. Genet. (2020), 29(17), 2831-2844
- Gervason S., Grandas A., Fontecave M., Schünemann V., Cianférani S., Sizun C., Tolédano M.B., D’Autréaux B.* Physiologically relevant reconstitution of iron-sulfur cluster biosynthesis uncovers persulfide-processing functions of ferredoxin-2 and frataxin Nature Commun. (2019), 10(1):3566.
Collaborators
– Christian SIZUN, ICSN Gif-Sur-Yvette, NMR spectroscopy
– Bénédicte BURLAT, Bruno GUIGLIARELLI, CNRS Marseille, EPR spectroscopy
– Volker SCHUNEMANN, Kaiserslautern University, Germany, Mössbauer spectroscopy
– Emiliano FONDA, Synchrotron SOLEIL, X-ray absorption spectroscopy
– Sarah CIANFERANI, Oscar HERNANDEZ IPHC Strasbourg, Mass spectrometry
– Yvain NICOLET, Michael CHERRIER IBS Grenoble, Anaerobic X-ray crystallography, cryo-EM
– David P. GOLDBERG, John Hopkins University, USA, Iron-Sulfur complex models,
– Mickael PLUTH, USA, Persulfide donors,
– Rafal DUTKIEWICZ, Gdansk University, Poland, E. coli system
– Javier MARTINEZ, Medical University of Vienna, Austria, Sulfur Metabolism
– Yves BOULARD, I2BC CEA Saclay, Modelling, Docking,
– Véronique MONNIER, Paris-Cité University, Drosophila FA model
Fundings

![]()