Year of commissioning : 2017
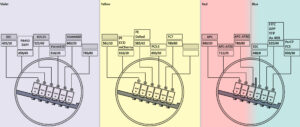
Lasers:
- 405 nm (4 detectors)
- 488 nm (2 detectors)
- 561 nm (4 detectors)
- 640 nm (3 detectors)
Holder:
- 5-ml tubes
- Eppendorf 2-ml tubes
- Eppendorf 1.5-ml tubes
- 96-wells plate
Up to 10,000 events per second
Software: CytExpert 2.5
Optical diagram available here