Intracellular Trafficking and Organelle Biogenesis
in Toxoplasma
Deciphering structures and functions of key parasite’s receptors involved in protein and lipid transport in T. gondii.
Unravel molecular functions of novel membrane binding factors that can finely regulate protein and lipid trafficking during secretory organelle biogenesis
Overview
Our progress in unraveling T. gondii intracellular trafficking inspired the idea that endosomal systems have been repurposed to form secretory organelles (Figure 1). Otherwise, the secretory organelle formation in the parasite relies on the functions of the transport of lipids and proteins from the Golgi to the endosomal-like comportment (ELC). This intracellular transport of proteins and lipids that are necessary for secretory organelle biogenesis requires key receptors that traffic vesicles containing ROP and MIC proteins to the destined organelles, rhoptries and micronemes, which are essential for host-parasite interaction, and parasite virulence. However, our knowledge of the biochemical mechanisms underlying the biogenesis of these secretory organelles remains to be understood.
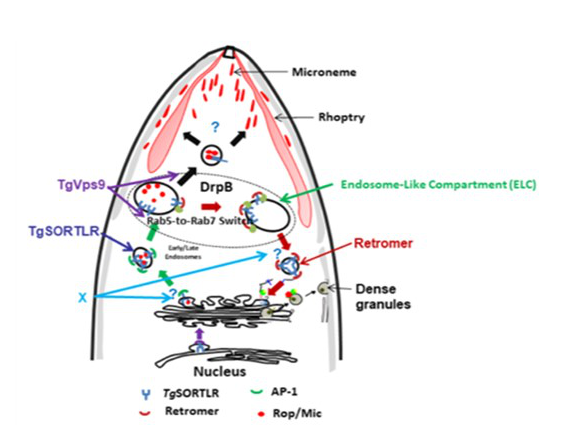
Figure 1: A novel strategy of merging the endosomal-like pathway and secretory systems for apical organelle biogenesis in T. gondii but also in other apicomplexan parasites. The different regulators (X) remain to be identified.
Our model systems
Toxoplasma gondii belongs to Apicomplexa that include life-threatening intracellular parasites, which have unusual secretory organelles, known to be essential for host infection. A major outstanding problem in understanding the pathogenicity of Apicomplexa is to determine the biogenesis of secretory organelles named rhoptries, micronemes and dense granules (Figure 2A). T. gondii relies on transport vesicles that accurately deliver virulence-like factors named ROP, MIC and GRA proteins to secretory organelles that are required for host cell entry and hijacking of many host functions (Figure 2A). During host cell entry, the parasite will build the parasitophorous vacuole (PV), which is a unique replicative niche for dividing intracellular parasites (Figure 2B). Derived from host plasma membrane, the vacuole is rendered nonfusogenic with the host endolysosomal system. T. gondii secretes numerous ROP and GRA proteins to modify the forming vacuole, enable nutrient uptake, and set up mechanisms of host subversion. The parasite will forme de novo secretory organelles, such as rhoptries and micronemes but also an inner membrane complex during daughter parasite (DC) formation (Figure 2C). Therefore, deciphering how intracellular trafficking signals mediate high fidelity sorting into these intracellular compartments represents a central challenge.
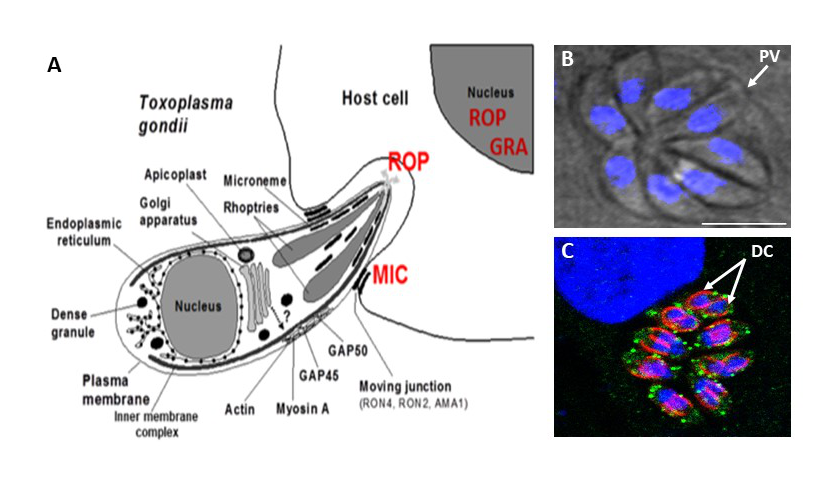
Figure 2: (A) Entry of Toxoplasma into the host cell requires the concerted actions of rhoptries, micronemes and dense granules that released ROP, MIC and GRA proteins. (B) After host cell entry, one parasite will replicate to eight daughter parasites at 24-hour post-infection, and this occurs within a niche named parasitophorous vacuole (PV). (C) Mode of replication of intracellular parasites (endodyogeny), leading to inner complex membrane and daughter cell (DC) formation (red signal) and accumulation of lipids as internal vesicles (green signals).
Research highlights
Our team studies the mechanisms involved in intracellular trafficking and secretory organelle biogenesis in T. gondii. We uncovered that transport of proteins to these organelles requires a unique receptor named TgSORTLR for T. gondii Sortilin-Like Receptor (Figure 3). TgSORTLR resides in a non-conventional endosomal-like compartment (ELC) and recruits MIC and ROP proteins via its luminal region. Importantly, no rhoptries and micronemes were formed in the mutants lacking TgSORTLR, leading to a complete inhibition of host invasion and mice infection (Figure 3). We also demonstrated that the C-terminal tail of TgSORTLR is required for binding to the retromer Vps35-Vps29-Vps26 and recycling from Rab5 to Rab7-dependent ELC before its delivery back to Golgi. T. gondii Vps9, a guanine nucleotide exchange factor (GEF) that regulates Rab5 is also crucial for TgSORTLR function. Jointly, our findings unveiled that TgSORTLR, TgVps9 and retromer are essential for trafficking protein complexes into secretory organelles (Figure 3).
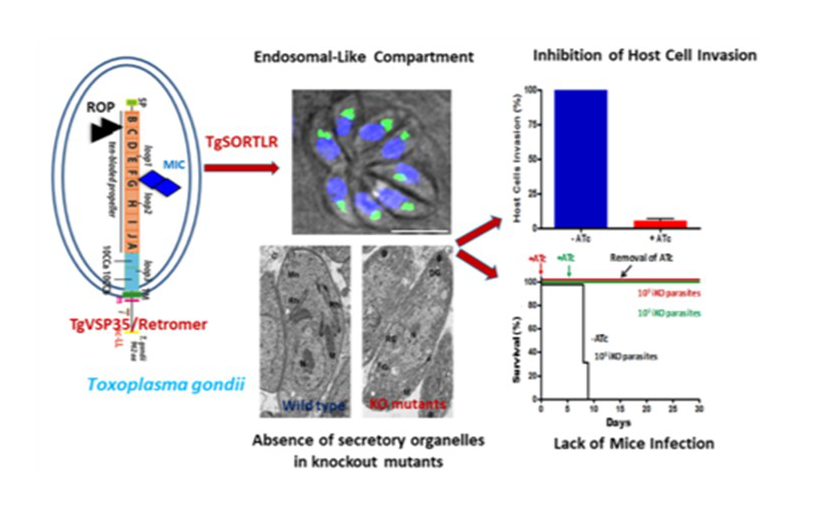
Figure 3: TgSORTLR functions require endosomal-like compartment and TgVSP35/retromer. Ablation of TgSORTLR and TgVSP35 led to the absence of secretory organelles and inhibition of infection.
Topics
We now envision using novel membrane binding factors as proof of concept that parasite-specific proteins can finely regulate protein and lipid trafficking during secretory organelle biogenesis. By combining biochemistry, cell and structural biology approaches, we aim :
- To determine the structure of TgSORTLR-ligand complexes
- To study lipid binding and membrane deformation during organelle biogenesis
- To decipher functions of the late secretory vesicles that drive organelle formation
In sum, because T. gondii is less complex than other mammalian systems, we expect to obtain mechanistic insights into how membrane interacting factors function in these parasites. As minute organisms branching off the eukaryotic lineage prior to the divergence of animals, fungi and plants, our studies may also provide new opportunities for therapeutic strategies.
team
Aissatou DIENG
Master Student
Ariane HONFOZO
PhD
Rodrigue HOUNGUE
PhD
Latest publications
For all the publications of the Team click on the button below.
External funding

Grant ANR-19-CE44-0006 (2019-2024)
Available post doctoral position
POST DOCTORAL POSITION – 24 months
Functional studies of novel cell trafficking factors in Toxoplasma gondii
A two-year postdoctoral position funded by the French National Research Agency (ANR) is available at the research group of « Intracellular Trafficking and Organelle Biogenesis of Toxoplasma » in the Institute for Integrative Biology of the Cell (I2BC), UMR 9198 CNRS-CEA, and Paris-Saclay University. Our group is interested in the intracellular trafficking of proteins and lipids that are involved in the biogenesis of secretory organelles known to play key roles in infection of hosts by Toxoplasma gondii. The current project will focus on the functional analyses of newly identified parasite’s proteins that bind to lipids, which are likely involved in the membrane trafficking from the post-Golgi/endosome-like compartement (ELC) to the secretory organelles of T. gondii. By using a combination of approaches including cell biology and biochemistry, we aim to decipher the roles of these novel proteins in the formation of membrane-bound vesicles that are required for secretory organelle biogenesis.
The applicant should have a strong background in cell and molecular biology. Prior experience in imaging (confocal microscopy) analyses and biochemical methods to study protein-protein (IP) will be highly advantageous. The ideal candidate should be highly motivated, curious and will be actively involved in interdisciplinary research with renowned laboratories and core facilities of the Institute for Integrative Biology of the Cell (I2BC). A good level in English (written and spoken) is required. Review of applications will begin immediately until the position filled.
Interested candidates should send by e-mail their CV, a list of two or three references and a cover letter stating their research experience and interests to :
Dr Stan Tomavo
Directeur de Recherche CNRS
Institut de Biologie Intégrative de la Cellule (I2BC)
Université Paris-Saclay – CEA – CNRS UMR 9198
Email : stanislas.tomavo@i2bc.paris-saclay.fr