2026
Summary
- Snapshots of cotranslational N-myristoylation reveal NMT as a ribosome-associated chaperone
- Crosstalk between cohesins and axis proteins determines meiotic chromosome architecture in Sordaria macrospora
- Flexizyme-Based Strategy for the Synthesis of Stable, Non-Isomerizable Amide-Linked 2’-Aminoacyl-tRNAs and their Shortened Analogs
- Impact of GC content on de novo gene birth
- Team of J. Soutourina received the label from Ligue Nationale contre le cancer
- Actomyosin-dependent assembly of the mechanosensitive machinery from adherens junctions triggers actin polymerization and organization
- The heme A synthase Cox15, as a target of redox-active 3-benzylmenadiones with antiparasitic activity
- How virulence genes reorganize the Salmonella genome
- Gain and loss of gene function shaped the nickel hyperaccumulation trait in Noccaea caerulescens
- Cross-regulation of iron-sulfur cluster biosynthesis by frataxin and ferredoxin-2
Snapshots of cotranslational N-myristoylation reveal NMT as a ribosome-associated chaperone
Nature Communications
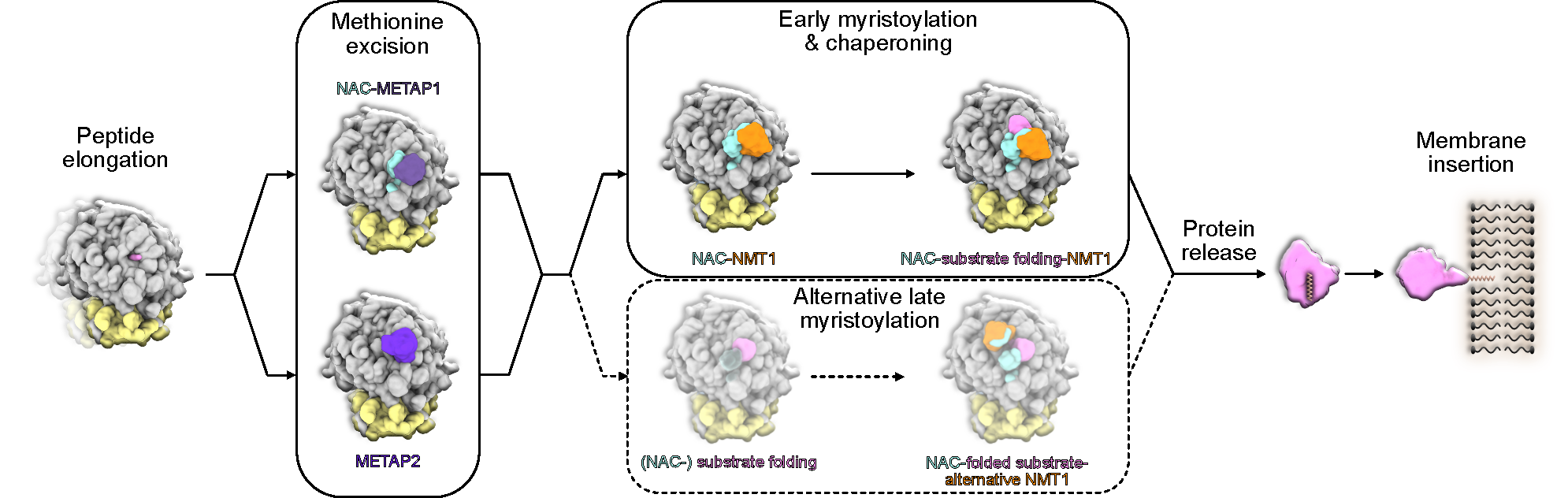
NMT in a new light: associated with the ribosome via the NAC complex, this enzyme not only adds lipid tags to the N-termini of nascent proteins but also acts as a chaperone, cooperating with MetAPs to ensure proper folding and delivery.
N-myristoylation is an essential cotranslational lipid modification catalyzed by N-myristoyltransferases (NMTs). Structural and cellular analyses reveal that NMT1 associates with the ribosomal tunnel exit via the nascent polypeptide–associated complex (NAC) and acts sequentially after MetAP-mediated initiator methionine removal, in contrast to previously described simultaneous cotranslational modification assemblies. Unexpectedly, NMT1 also exhibits chaperone-like activity, expanding its functional repertoire in cotranslational protein biogenesis.
More information: https://www-nature-com.insb.bib.cnrs.fr/articles/s41467-025-67962-4
Contacts: Thierry MEINNEL <thierry.meinnel@i2bc.paris-saclay.fr> and Carmela GIGLIONE <carmela.giglione@i2bc.paris-saclay.fr>
Crosstalk between cohesins and axis proteins determines meiotic chromosome architecture in Sordaria macrospora
Plos Genetics
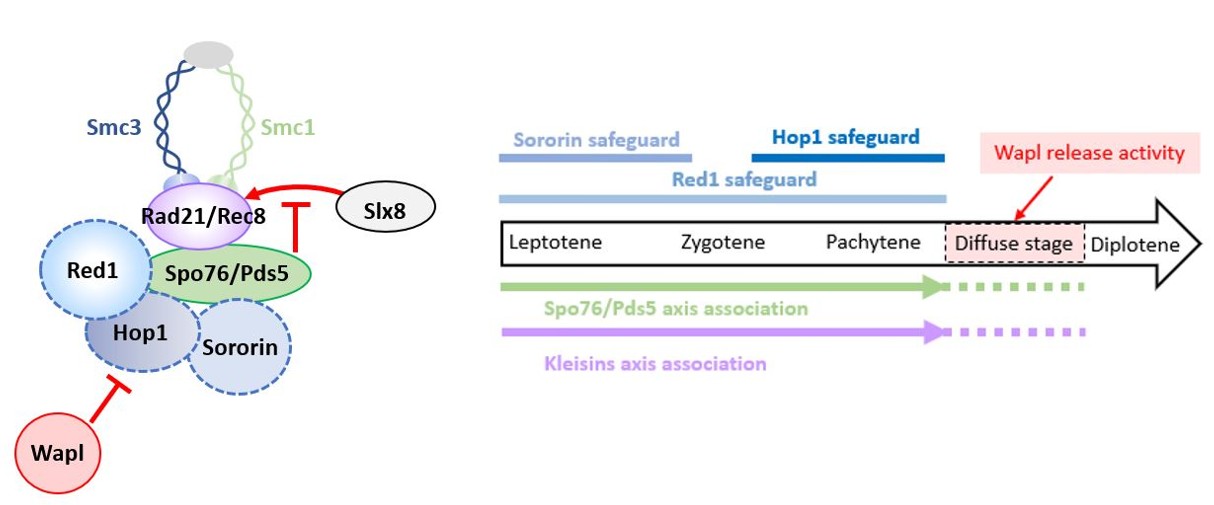
Discovery: a dynamic interplay between axis proteins and cohesins ensures chromosome stability during meiosis in Sordaria.
Faithful chromosome segregation during meiosis requires the coordinated action of cohesin complexes and chromosome axis proteins. How these factors interact and communicate along chromosome axes, especially during meiotic prophase I, remains however, only partially understood. We therefore investigated the functional interplay between the cohesin components and regulators (Rad21, Rec8, Wapl, Sororin, Spo76/Pds5) and two meiosis-specific axis proteins Red1 and Hop1. Analysis of multiple combinations of their corresponding null mutants and of their genetic-epistasis interactions in the fungus Sordaria macrospora revealed a hierarchical regulatory network for their recruitment and releasing. This work uncovers an unexpected role of axis proteins Red1 and Hop1, that together with Sororin, provide stage-specific protection of Spo76/Pds5 against Wapl-mediated release. Furthermore, we identify that Spo76/Pds5 is the main target of Wapl and acts as a central guardian of kleisin stability against Slx8/STUbL-dependent proteasomal degradation. Together, our findings show that dynamic crosstalk between axis proteins and cohesins is crucial to preserve axis integrity and to ensure accurate meiotic progression.
More information: https://pubmed-ncbi-nlm-nih-gov.insb.bib.cnrs.fr/41433359/
Contact: Stéphanie BOISNARD <stephanie.boisnard@i2bc.paris-saclay.fr>
Flexizyme-Based Strategy for the Synthesis of Stable, Non-Isomerizable Amide-Linked 2’-Aminoacyl-tRNAs and their Shortened Analogs
Chemistry Europe
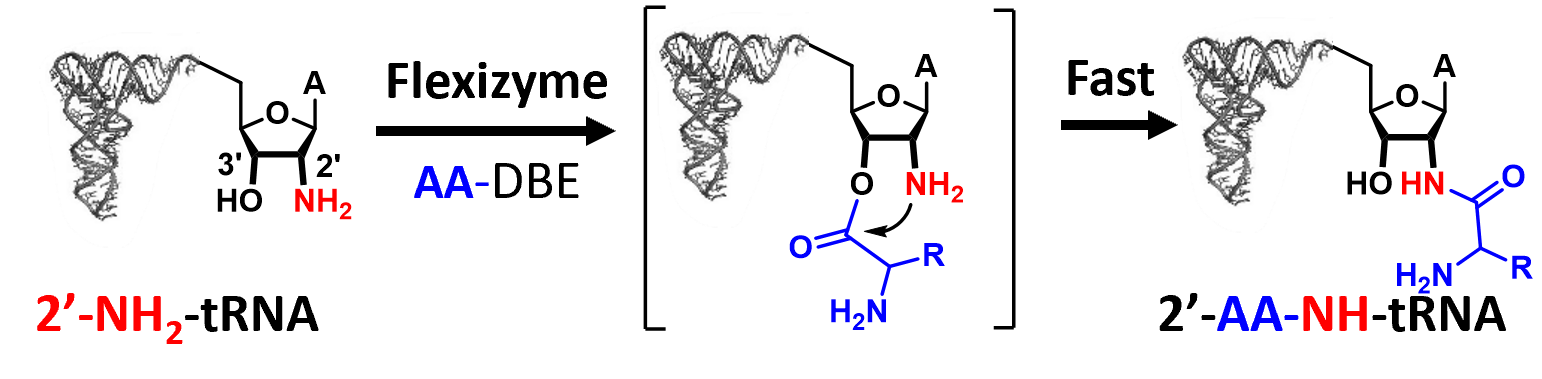
We developed a flexizyme-based semi-synthetic strategy that provides access to stable 2′- and 3′-amide AA-tRNA analogs, offering robust tools for structural biology and for probing regiospecificity in AA-tRNA-dependent enzymes.
The study of the regiospecificity of aminoacyl-tRNA (AA-tRNA)-dependent enzymes and their structural characterization with AA-tRNAs are limited by rapid hydrolysis of the ester bond linking amino acid to tRNA. To overcome this limitation, stable AA-tRNA analogs bearing hydrolysis-resistant linkages, such as amide bonds or ester bioisosteres, have been developed. These analogs are valuable tools for investigating interactions between AA-tRNAs and various enzymes or ribonucleoproteins, including elongation factors, ribosomes, Fem-family transferases, and cyclodipeptide synthases. However, their synthesis remains technically challenging. Recently, flexizymes—engineered ribozymes capable of aminoacylating tRNAs with diverse amino acids or analogs—have enabled the synthesis of 3′-amide-linked AA-NH-tRNAs. Due to their inherent specificity for 3′-OH acylation, flexizymes have not been used to generate 2′-amide-linked analogs, and such regioisomers have remained unexplored. In this study, we demonstrate that while flexizymes cannot directly aminoacylate the 2′ position, they can nevertheless mediate the synthesis of 2′-aminoacyl-NH-tRNAs via a two-step regioisomerization mechanism with excellent yields. This finding provides new insights into the binding mode of AA-tRNAs to flexizymes and expands the chemical space of stable AA-tRNA analogs. Access to both 3′- and 2′-amide regioisomers will enable more precise studies of AA-tRNA recognition and catalysis by various AA-tRNA-dependent systems.
More information: https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202503506
Contact: Matthieu FONVIELLE <matthieu.fonvielle@i2bc.paris-saclay.fr>
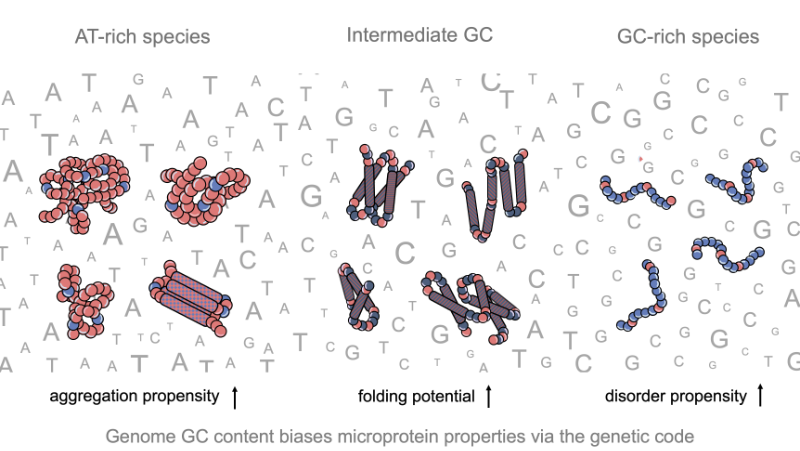
Impact of GC content on de novo gene birth
Nature Communications
Noncoding DNA can generate microproteins, some of which evolve into new genes. We show that de novo genes preferentially originate from GC-rich, foldable sequences, revealing how base composition channels the birth of new proteins.
Noncoding regions of eukaryotic genomes are widely transcribed and constitute a major source of novel microproteins, some of which eventually become fixed as de novo genes – a process known as de novo gene birth that plays a significant role in species adaptation. However, the structural properties of these nascent proteins and the factors governing their evolutionary fate remain poorly understood. In particular, the role of genome nucleotide composition (GC content) in shaping their biophysical properties has remained unclear. Here, we analyzed the foldability and sequence properties of millions of putative microproteins encoded by intergenic open reading frames (ORFs) from 3,379 eukaryotic species spanning a broad range of GC contents (18–79%). We show that GC content strongly influences amino-acid composition and structural tendencies, suggesting distinct cellular impacts if non-genic regions are pervasively expressed. AT-rich species predominantly encode ORFs biased toward hydrophobic, aggregation-prone sequences, whereas GC-rich species tend to encode more hydrophilic, disorder-prone ORFs. ORFs from genomes with intermediate GC content display a more balanced composition and higher folding potential, with many expected to adopt proto-folds. To assess how these properties relate to gene emergence, we traced the evolutionary history of several hundred de novo proteins across 22 species using phylostratigraphy, targeted de novo gene searches, and ancestral sequence reconstruction. We find that de novo genes preferentially originate from GC-rich ORFs with intrinsic folding potential. Together, our results reveal that the interplay between GC content and foldability – rooted in the structure of the genetic code – shapes the emergence of novel genes.
More information: https://www.nature.com/articles/s41467-025-68022-7
Contact: Anne LOPES <anne.lopes@i2bc.paris-saclay.fr>
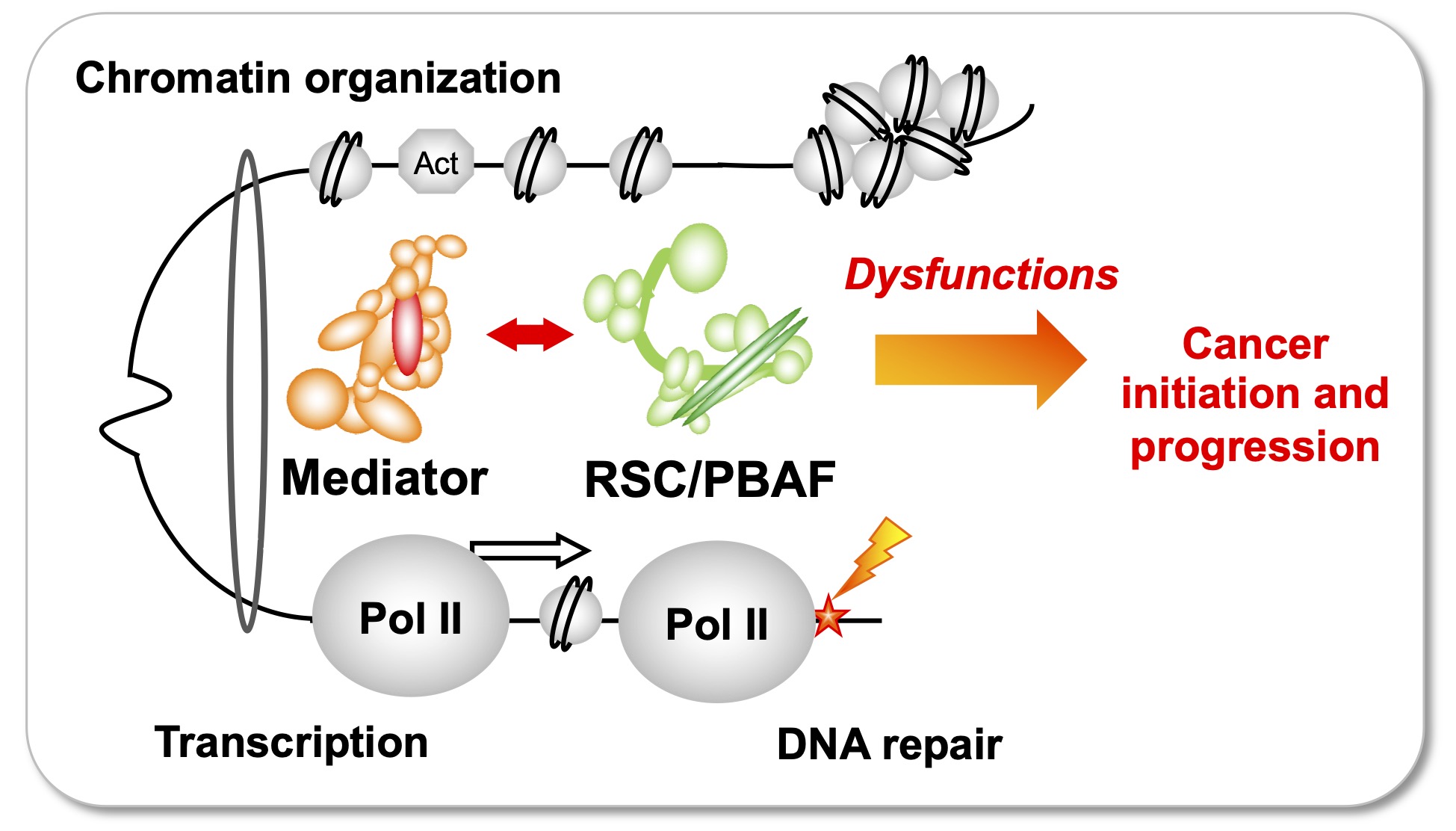
Understand how the essential complexes, the coregulator Mediator and the chromatin remodeling complex RSC/PBAF, cooperate in the nucleus.
In eukaryotes, transcription and DNA repair occur in the crowded context of chromatin. Dysfunctions of these processes can lead to cancers. Mediator is an essential and conserved multisubunit coactivator complex, mutated in many cancers. However, it remains largely unknown how Mediator and chromatin regulators coordinate their functions. Our recent publication suggests the novel hypothesis that Mediator acts in conjunction with the chromatin remodeling complex RSC (Remodels the Structure of Chromatin) of SWI/SNF family, homologous to PBAF (Polybromo-associated BAF) in human, representing the most frequently mutated complexes in cancers. In this project supported by Ligue Nationale contre le cancer, using the yeast model with a perspective to extend the study to human cells, we intend to decipher the molecular mechanisms involved in functional cooperation between these essential coregulator complexes in transcription regulation, DNA repair and chromatin organization relevant for cancer biology.
Contact: Julie SOUTOURINA <julie.soutourina@i2bc.paris-saclay.fr>

Actomyosin-dependent assembly of the mechanosensitive machinery from adherens junctions triggers actin polymerization and organization
ScienceAdvances
Adherens junction mechanosensing.
To form tissues, cells adhere to one another through structures known as adherens junctions. These junctions have the ability to adapt to the mechanical variations to which tissues are subjected, thereby preserving their integrity.
In this study published in Science Advances, we studied this mechanism by combining various biochemical approaches, including an unprecedented in vitro reconstitution involving surface micropatterning and up to eight purified proteins.
We show that contraction of the actomyosin cytoskeleton is sufficient to trigger the assembly of three key proteins—α-catenin, vinculin, and VASP. Once associated, these proteins cooperate to polymerize and reorganize actin.
These findings reveal how adherens junctions sense and adapt to mechanical forces exerted by the cytoskeleton by strengthening their connection to it, thereby contributing to tissue cohesion and stability..
More information: https://www.science.org/doi/10.1126/sciadv.ady4863
Contact: Christophe LE CLAINCHE <christophe.leclainche@i2bc.paris-saclay.fr>

The heme A synthase Cox15, as a target of redox-active 3-benzylmenadiones with antiparasitic activity
Antimicrobial Agents and Chemotherapy
We identified a new target for antiparasitic compounds and it is in the mitochondria.
Chagas disease, caused by Trypanosoma cruzi, is a neglected parasitic infection. The very limited arsenal of anti-T. cruzi treatments calls for the development of new drugs. Recently, a library of 3-benzylmenadione derivatives was synthesized with cruzidione being the most efficient and specific compound against the parasite. To decipher its mode of action, we used the yeast Saccharomyces cerevisiae as model. Evidence pinpointed at the heme A synthase Cox15 as a primary target of cruzidione: 1) a mutation in Cox15 (i.e., S429F) renders the yeast cells highly sensitive to the drug, 2) treatment with cruzidione led to the loss of cytochrome c oxidase, an enzyme that relies on heme A as an essential cofactor and 3) replacement of the yeast Cox15 by T. cruzi enzyme resulted in a high sensitivity to cruzidione. We then investigated the effect of cruzidione in T. cruzi and observed a significant reduction of heme contents, most likely involving the inhibition of the heme A synthase. This, in turn, led to a decrease in O2 consumption by the parasite. Finally, using the yeast model, we showed that, similarly to what we previously found for the antimalarial benzylmenadione plasmodione, NADH-dehydrogenase plays a key role in cruzidione bioactivation. We proposed that the reduced benzoylmenadione metabolites produced by the reaction with NADH-dehydrogenase, act as Cox15 inhibitors. This study, through the identification of the mode of action of cruzidione, highlighted Cox15 as a novel target for antiparasitic drugs.
More information: https://journals.asm.org/doi/10.1128/aac.01161-25
Contact: Brigitte MEUNIER <brigitte.meunier@i2bc.paris-saclay.fr>

How virulence genes reorganize the Salmonella genome
Nature Communications
Using functional genomics in sorted Salmonella populations and high-resolution microscopy, the authors show that the expression of Pathogenicity Island 1 is associated with chromatin remodeling and with the repositioning of this region toward the nucleoid periphery.
Chromatin provides a universal framework for organizing and regulating genomes across the three domains of life. In bacteria, it is composed of intrinsically supercoiled DNA associated with small DNA-binding proteins known as nucleoid-associated proteins (NAPs). Their binding can induce DNA bending, bridging, coating, and/or wrapping, giving rise to distinct modes of chromatin organization.
Bacterial chromatin can exist in a repressed state (silent chromatin) or in an actively transcribed state (active chromatin). Silent chromatin is largely associated with H-NS, a xenogeneic silencer that restricts the costly expression of genes acquired by horizontal transfer. In contrast, active chromatin is densely occupied by RNA polymerase and is characterized by different levels of DNA supercoiling. However, the changes in protein occupancy and chromatin organization that accompany transitions between these two states remain poorly understood.
Researchers of the Genome Biology Department of the I2BC in collaboration with the NGS and the Imaging facility of the I2BC and the Trinity College Dublin (Ireland), have unveiled the chromatin organization of horizontally acquired regions in Salmonella enterica serovar Typhimurium, which are essential for its pathogenicity. They show that expression of Pathogenicity Island 1 (SPI-1) is accompanied by local chromatin remodeling, marked by profound changes in three-dimensional organization and protein occupancy. This remodeling is also associated with the repositioning of SPI-1 toward the nucleoid periphery.
These findings provide new insights into the interplay between xenogeneic silencing, counter-silencing mechanisms, chromatin architecture, and the evolutionary integration of acquired DNA. They also reveal a finely tuned chromatin remodeling process that minimizes the cellular cost of activating pathogenicity islands, and they establish a direct link between the linear (1D) organization of the genome and its three-dimensional (3D) folding.
More information: https://www.nature.com/articles/s41467-025-67746-w
Contact: Virginia LIOY <virginia.lioy@i2bc.paris-saclay.fr>

Gain and loss of gene function shaped the nickel hyperaccumulation trait in Noccaea caerulescens
The Plant Cell
Plants that hyperaccumulate nickel open the possibility to mine this metal from soils in an environmetally friendly manner. In this study, sequencing of a nickel hyperaccumulating plant together with genomic and transcriptomic comparisons reveal the molecular mechanisms underlying this extreme trait.
Nickel hyperaccumulation is an extreme adaptation to ultramafic soils observed in more than 500 plant species. However, our
understanding of the molecular mechanisms underlying the evolution of this trait remains limited. To shed light on these
mechanisms, we have generated a high-quality genome assembly of the metal hyperaccumulator Noccaea caerulescens. We then used
this genome as reference to conduct comparative intraspecific and interspecific transcriptomic analyses using various accessions of
N. caerulescens and the non-accumulating relative Microthlaspi perfoliatum to identify genes associated with nickel hyperaccumulation.
Our results suggest a correlation between nickel hyperaccumulation and a decrease in the expression of genes involved in defense
responses and the regulation of membrane trafficking. Surprisingly, these analyses did not reveal a significant enrichment of genes
involved in the regulation of metal homeostasis. However, we found that the expression levels of selected metal transporter genes,
namely, NcHMA3, NcHMA4, and NcIREG2, are consistently elevated in N. caerulescens accessions hyperaccumulating nickel.
Furthermore, our analyses identified frameshift mutations in NcIRT1 associated with the loss of nickel hyperaccumulation in a few
accessions. We further showed that the expression of a functional NcIRT1 in the roots of the La Calamine accession increases nickel
accumulation in shoots. Our results demonstrate that NcIRT1 participates in nickel hyperaccumulation in N. caerulescens. They also
suggest that nickel hyperaccumulation is an ancient trait in N. caerulescens that has evolved from the high and constitutive expression
of several metal transporters, including NcIREG2, and that the trait was subsequently lost in a few accessions due to mutations in NcIRT1.
More information: https://doi.org/10.1093/plcell/koaf281
Contact: Sébastien THOMINE <sebastien.thomine@i2bc.paris-saclay.fr>

Cross-regulation of iron-sulfur cluster biosynthesis by frataxin and ferredoxin-2
Nature
Tight regulation of Fe-S clusters biosynthesis via a mutually antagonistic binding of frataxin and ferredoxin-2 to the assembly machinery, with several important implications for the Friedreich’s ataxia disease caused by frataxin deficiency.
Iron-sulfur (Fe-S) clusters are essential metallocofactors that perform a multitude of biological functions. They are synthesized de novo by multi-proteins machienries and any defect in their synthesis leads to severe diseases such as Friedreich’s ataxia (FRDA), caused by defective expression of frataxin (FXN). Here, we uncover that efficient [2Fe-2S] cluster assembly requires a fine-tuned balanced ratio of FXN and Ferredoxin-2 (FDX2), an essential enzyme of the assembly process. [2Fe-2S] clusters are assembled on the scaffold protein ISCU2 with sulfur provided as a persulfide by NFS1, which is cleaved into sulfide by FDX2. FXN stimulates the whole process by accelerating persulfide transfer to ISCU2. Using an in vitro reconstituted human system, we show that any deviation from a close-to-equal amount of FXN or FDX2 downregulates Fe-S cluster synthesis. We performed a structure-function investigation, which revealed that this is due to competition between FXN and FDX2 for the same binding site on the NFS1-ISCU2 complex. We found that higher levels of FXN impair the persulfide-reductase activity of FDX2 and higher levels of FDX2 slow FXN-accelerated persulfide transfer to ISCU2. We also discovered that FDX2 directly hinders persulfide generation and transfer to ISCU2 by interacting with the persulfide-carrying mobile loop of NFS1. We further found that knocking-down FDX2 expression in a FRDA drosophila model, increases fly lifespan. Altogether, this work highlights a direct regulation of Fe-S cluster biosynthesis through antagonistic binding of FXN and FDX2 and suggests that decreasing FDX2 in the context of FXN deficiency in FRDA might constitute a novel therapeutic axis.
More information: https://www.nature.com/articles/s41586-025-09822-1
Contact: Benoit D’AUTRÉAUX <benoit.dautreaux@i2bc.paris-saclay.fr>